Preparation method of isoxepac
A technology of isoket acid and phenylacetic acid, applied in the direction of organic chemistry, can solve the problems of non-compliance with energy saving and emission reduction, green, safe production, risk of environmental damage, complex process route, etc., and achieve performance such as improving purity and appearance , reduce energy consumption and discharge of three wastes, and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
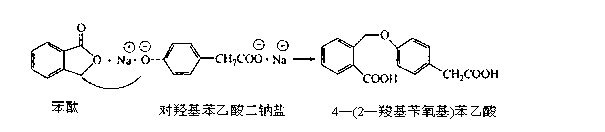
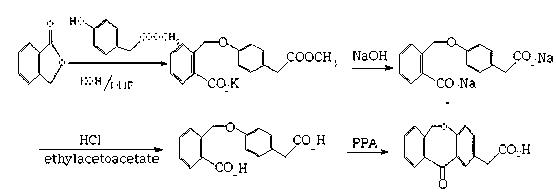
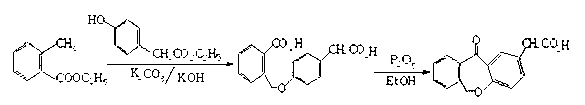
[0038] (1) Condensation: Prepare and weigh 10kg of p-hydroxyphenylacetic acid, 9kg of phthalide, and 10 kg of sodium methoxide, dissolve p-hydroxyphenylacetic acid and phthalide with 20kg of DMAC, add sodium methoxide, and then heat to 110°C for 7 hours at a pressure of 2 Pa. , rectification reclaims DMAC solvent, adds the hydrochloric acid solution that HCl content is 9-10wt% and adjusts the pH value to 3-4, crystallizes out 4-(2-carboxybenzyloxy)phenylacetic acid 17.8kg;
[0039] (2) Cycling: Dissolve the obtained 4-(2-carboxybenzyloxy)phenylacetic acid in 15kg of glacial acetic acid, then add 50 parts by weight of polyphosphoric acid, heat to 80°C for 8 hours at a pressure of 2Pa, and then add 5°C The sodium chloride solution cooling crystallization obtains 22kg of isoket acid crude product;
[0040] (3) Purification: Weigh 40 parts by weight of ethyl acetate, heat and dissolve the crude isoket acid, add 1.8kg of activated carbon for decolorization, and blow dry at a temperat...
Embodiment 2
[0046] (1) Condensation: Weigh 8kg of p-hydroxyphenylacetic acid, 6kg of phthalide, and 8kg of sodium methoxide, dissolve the p-hydroxyphenylacetic acid and phthalide with 16kg of DMAC, add the sodium methoxide, and then heat to 80 at a pressure of 0.1Pa. React at ℃ for 3 hours, rectify and recover the DMAC solvent, add a hydrochloric acid solution with an HCl content of 9-10wt% to adjust the pH value to 1, and crystallize 14.5 kg of 4-(2-carboxybenzyloxy)phenylacetic acid;
[0047] (2) Cycling: Dissolve the obtained 4-(2-carboxybenzyloxy)phenylacetic acid in 2kg of glacial acetic acid, then add 3kg of polyphosphoric acid, heat to 30°C for 3h at a pressure of 0.1Pa, then add 2°C Sodium chloride solution cooling crystallization obtains 20kg of isoket acid crude product;
[0048] (3) Purification: Weigh 25kg of ethyl acetate, heat and dissolve the crude isoket acid, add 0.9kg of activated carbon for decolorization, and vacuum dry at a temperature of 30°C to obtain 11.8kg of isok...
Embodiment 3
[0051] (1) Condensation: Weigh 12kg of p-hydroxyphenylacetic acid, 12kg of phthalide, and 12kg of sodium methoxide, dissolve the p-hydroxyphenylacetic acid and phthalide with 25kg of DMAC, add the sodium methoxide, and then heat to 170°C at a pressure of 10Pa React for 10 hours, recover the DMAC solvent by rectification, add a hydrochloric acid solution with an HCl content of 9-10wt% to adjust the pH value to 5, and crystallize 21.6 kg of 4-(2-carboxybenzyloxy)phenylacetic acid;
[0052] (2) Cycling: Dissolve the obtained 4-(2-carboxybenzyloxy)phenylacetic acid with 17kg glacial acetic acid glacial acetic acid, then add 52kg polyphosphoric acid, heat to 100°C under a pressure of 10Pa to react for 12h, then add 20°C The sodium chloride solution cooling crystallization obtains 28kg of isoket acid crude product;
[0053] (3) Purification: Weigh 50kg of ethyl acetate, heat and dissolve the crude isoket acid, add 8.4kg of activated carbon for decolorization, and vacuum dry at a tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com