Polysaccharide conjugation with detoxified E. COLI heat labile enterotoxin (LT) used as vaccine
A heat-resistant enterotoxin and Escherichia coli technology, applied in the direction of bacteria, antibacterial drugs, bacterial antigen components, etc., can solve problems affecting immunogenicity and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] It has now been found that the conjugated polysaccharide-LTS61K vaccine prepared according to the present invention can surprisingly induce higher polysaccharide-specific IgG antibody titers and greater bactericidal activity in serum than polysaccharides or polysaccharides mixed with LTS61K .
[0033] The list of abbreviations used in this article is as follows:
[0034] CFU: colony forming unit
[0035] ELISA: Enzyme-Linked Immunosorbent Assay
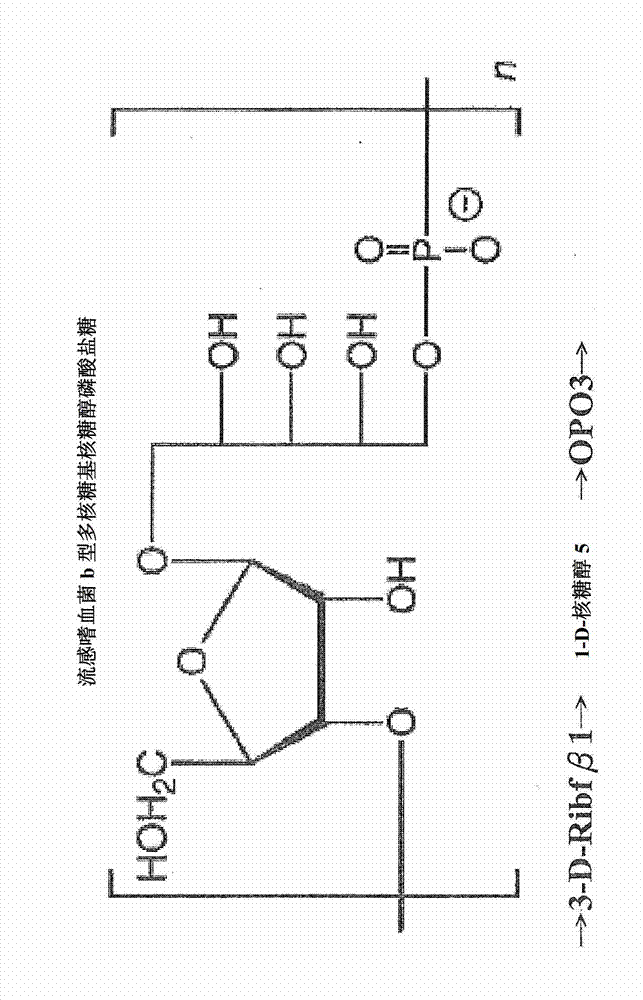
[0036] Hib: Haemophilus influenzae type b
[0037] IEF: Isoelectric Focusing
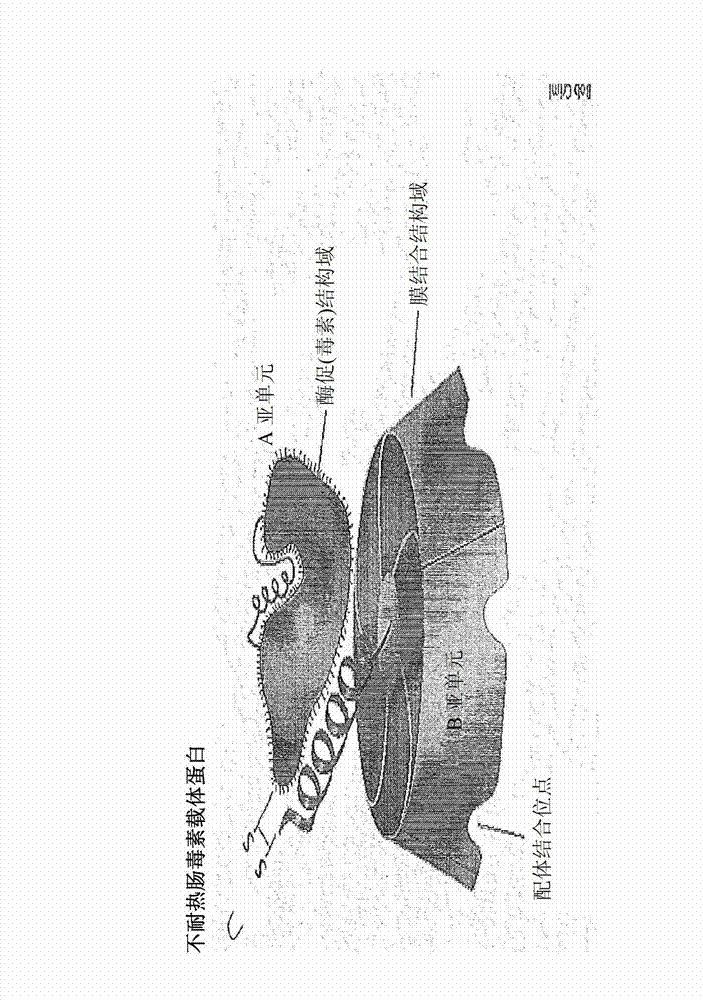
[0038] LT: heat-labile enterotoxin
[0039] MALLS: Multi-Angle Laser Scattering
[0040] OD: optical density
[0041] PNPS: pneumococcal polysaccharide
[0042] PRP: polyribosyl ribitol phosphate
[0043] PS: polysaccharide
[0044] SDS-PAGE: Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
[0045] SEC_HPLC: Size Exclusion High Pressure Liquid Chromatography
[0046] RI: reflectivity
[0047] The new carrier protein used in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com