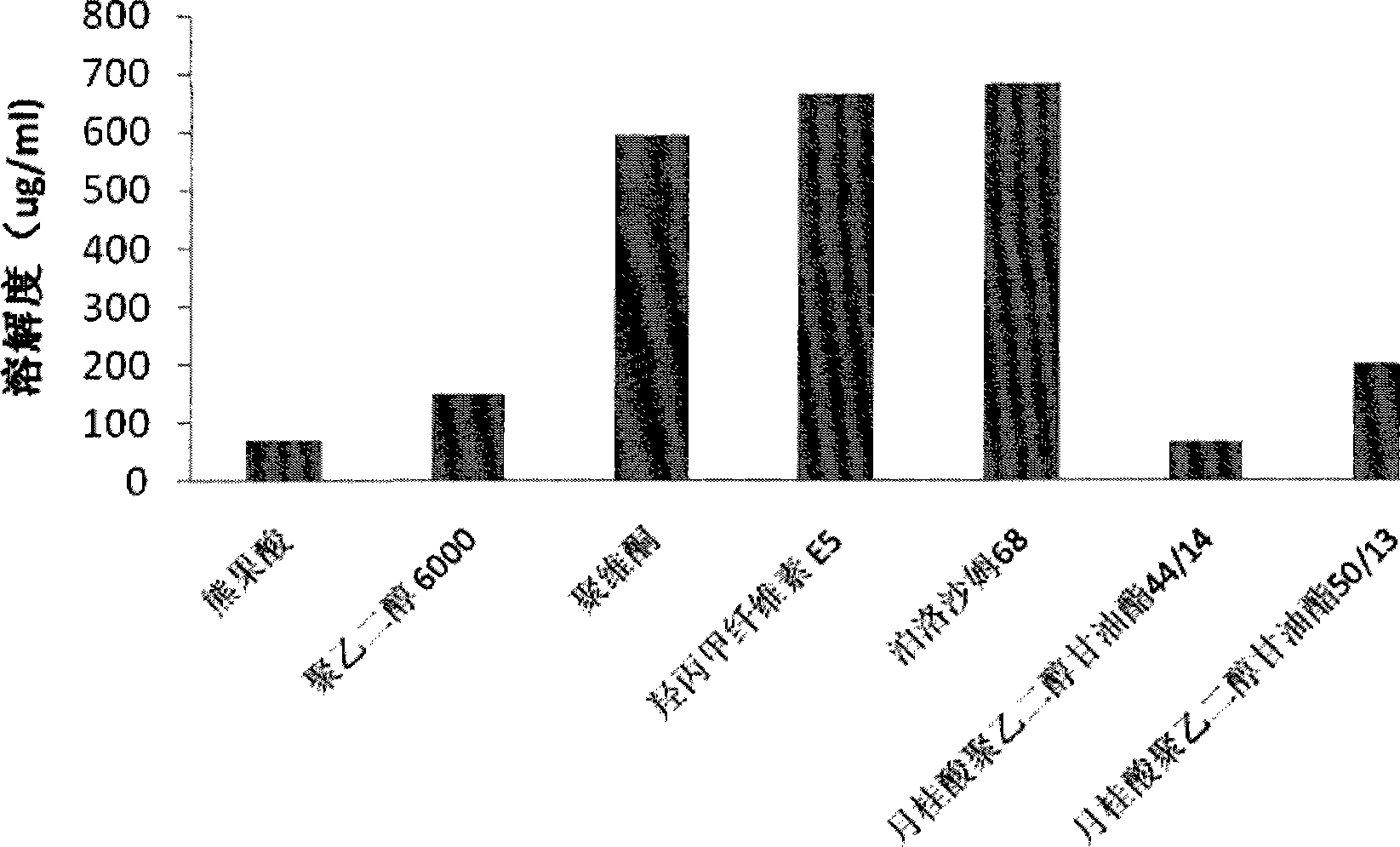
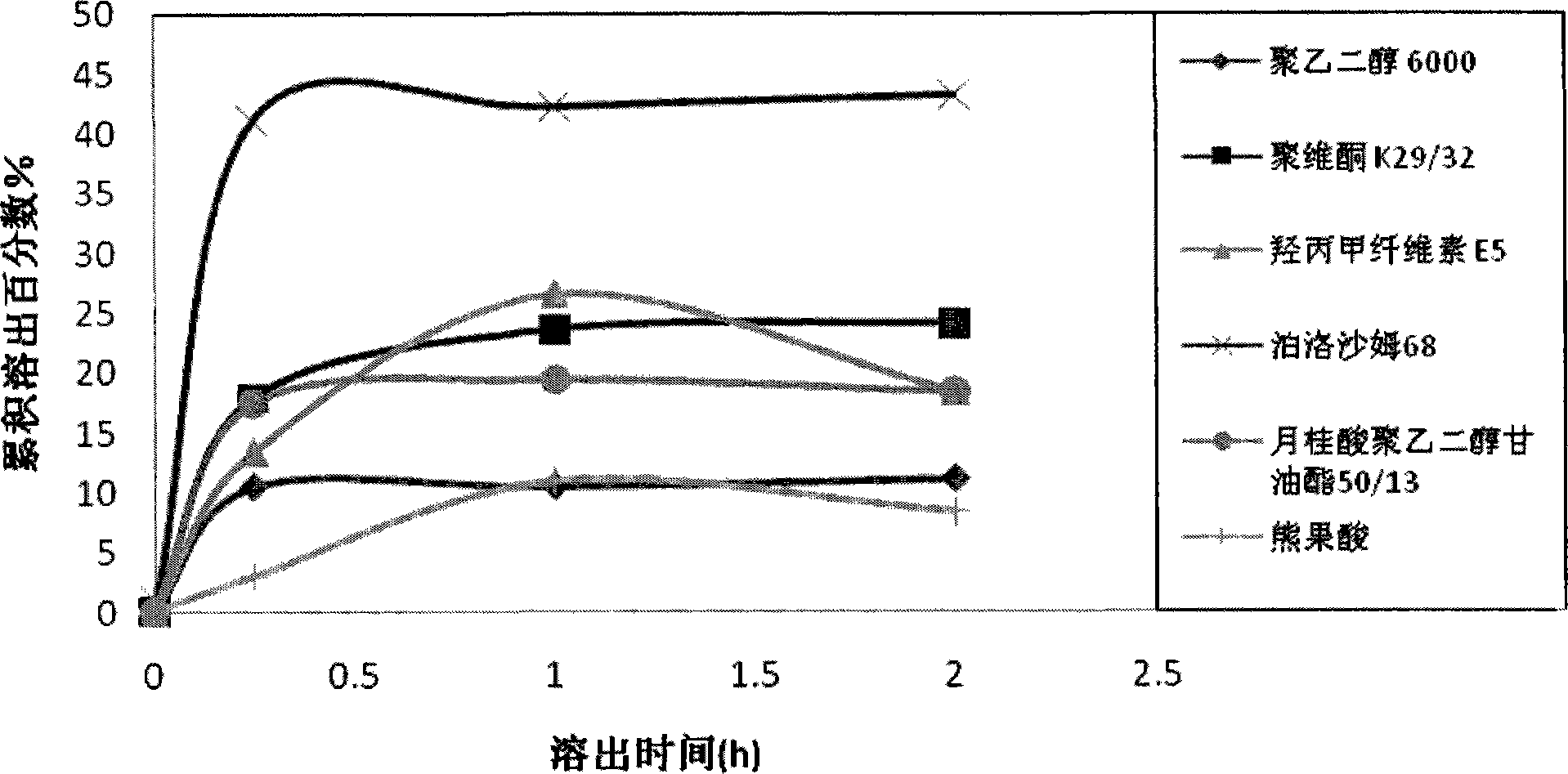
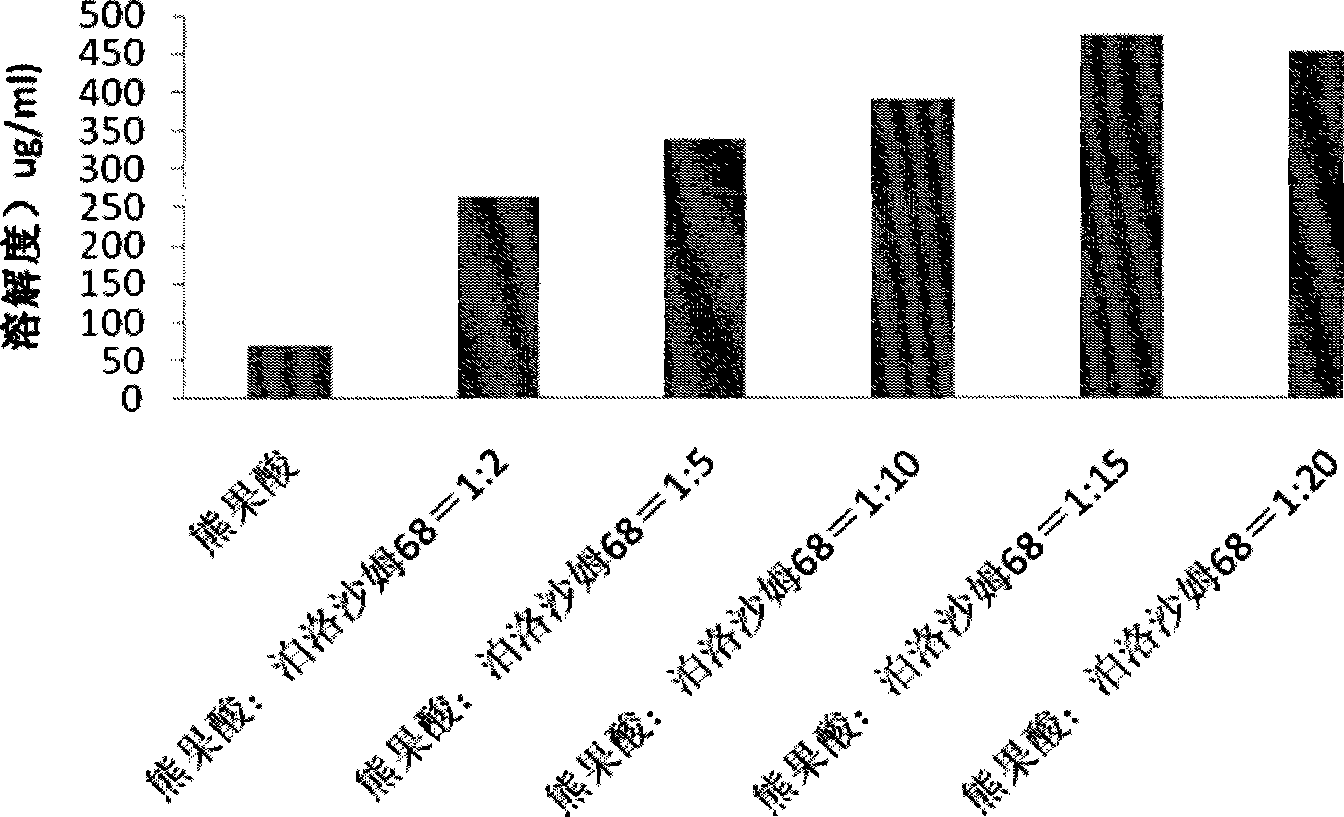
Ursolic acid solid dispersion and preparation method thereof
A technology of solid dispersion and ursolic acid, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of low bioavailability, complicated preparation process, and water solubility of ursolic acid. It can solve problems such as poor properties, and achieve the effects of broad market prospects, high solubility and good in vitro dissolution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Take 10g of ursolic acid and 150g of polyethylene glycol 6000, put them in a 10L bottle, add 4L of ethanol to ultrasonically dissolve, and use Gene Vac vacuum centrifugation to remove the organic solvent. The temperature control range is 30°C, and the concentration time is 2h. Place it in a vacuum oven at ℃ for 12 hours to remove the residual organic solvent, take it out, grind it through a 10-200-mesh sieve, and obtain it.
Embodiment 2
[0038] Take 10g of ursolic acid and 150g of povidone k29 / 33, put them in a 10L bottle, add 4L of ethanol to ultrasonically dissolve, and use Gene Vac vacuum centrifugation to remove the organic solvent. The temperature control range is 40°C, and the concentration time is 3h. Take out, Put it in a vacuum oven at 30°C for 14 hours to remove the residual organic solvent, take it out, grind it through a 10-200-mesh sieve, and obtain it.
Embodiment 3
[0040] Take 10g of ursolic acid and 150g of hypromellose E5, put them in a 10L bottle, add 4L of ethanol to ultrasonically dissolve, use Gene Vac vacuum centrifugation to remove the organic solvent, the temperature control range is 50°C, the concentration time is 12h, take out, Put it in a vacuum oven at 50°C for 16 hours to remove the residual organic solvent, take it out, grind it through a 10-200-mesh sieve, and obtain it.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com