Medicine osmotic pump preparation
An osmotic pump and drug technology, applied in the directions of osmotic delivery, drug delivery, pill delivery, etc., to achieve the effect of controllable drug release rate and complete drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] (1) Tablet core prescription:
[0060]
[0061] (2) Prescription of semi-permeable membrane coating solution:
[0062]
[0063] (3) Preparation process: Weigh each component according to the prescription amount, mix them evenly, and compress the tablet; coat the tablet core with acetone solution of cellulose acetate-PEG4000 until the weight of the tablet core increases by 4%; A small hole with a diameter of 0.8 mm was punched on one side of the sheet.
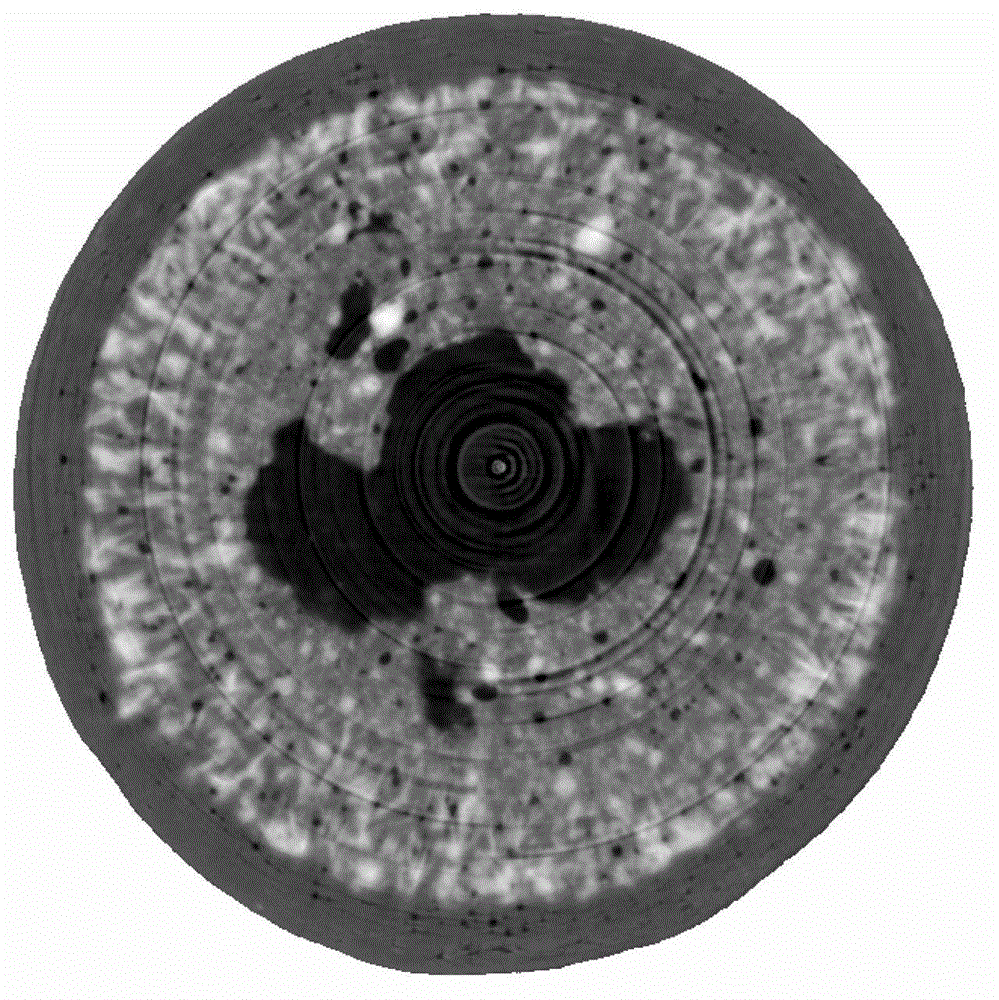
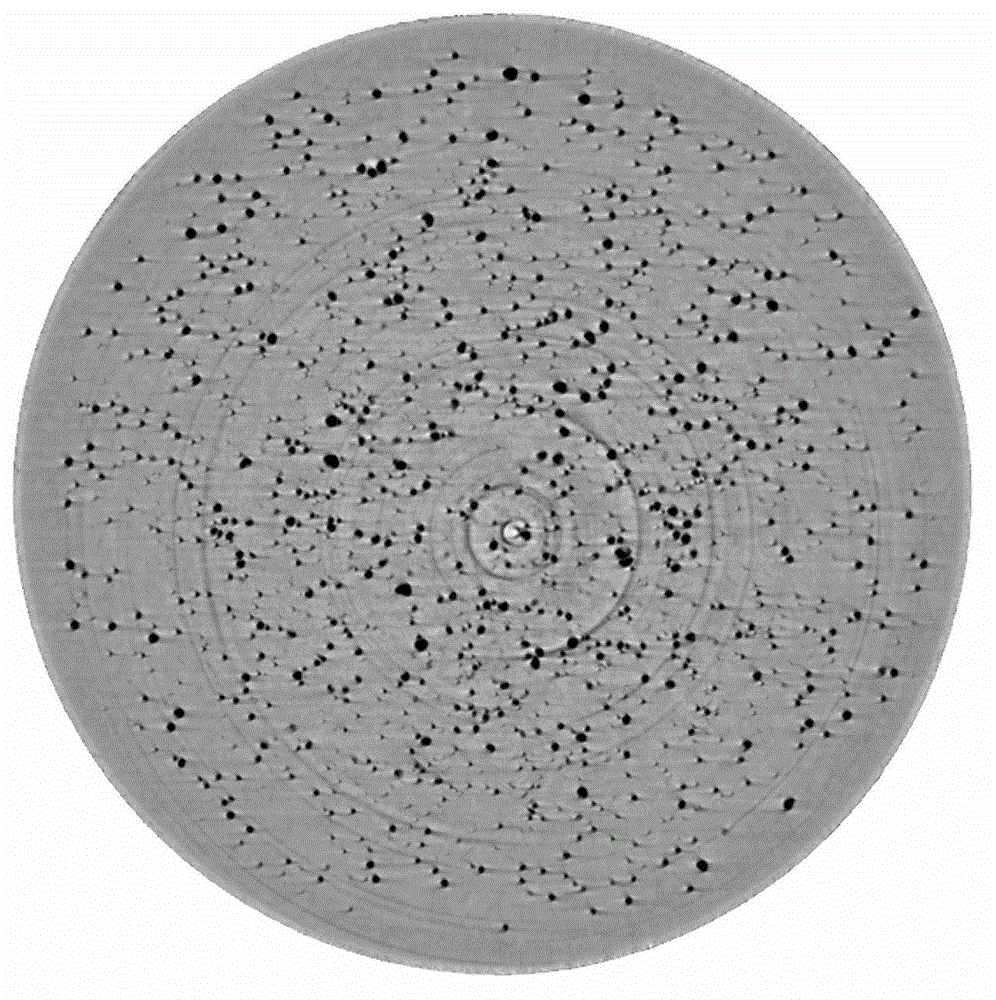
[0064] (4) Measuring method: Paddle method is adopted, 900mL degassed distilled water is used as release medium, and the rotating speed is 75 revolutions per minute. The release degree at different time points was measured, and the osmotic pump tablets were taken out at different dissolution time points, sealed in a desiccator filled with a desiccant (silica gel) at room temperature, and placed for 48 hours. Acquire two-dimensional images with a 180-degree viewing angle to complete SR-μCT three-dimensional scanni...
Embodiment 2
[0067] (1) Tablet core prescription:
[0068]
[0069]
[0070] (2) Prescription of semi-permeable membrane coating solution:
[0071]
[0072] (3) Preparation process: Weigh the components that have passed through a 100-mesh sieve according to the prescription and mix them evenly; finally add the prescribed amount of magnesium stearate, mix evenly, and press into tablets; Coating until the weight gain of the tablet core is 4%; use a laser to punch a small hole with a diameter of 0.8 mm on one side of the coated tablet.
[0073] (4) Measuring method: Paddle method is adopted, 900mL degassed distilled water is used as release medium, and the rotating speed is 75 revolutions per minute. The release degree at different time points was measured, and the osmotic pump tablets were taken out at different dissolution time points, sealed in a desiccator filled with a desiccant (silica gel) at room temperature, and placed for 48 hours. Acquire two-dimensional images with a 180-...
Embodiment 3
[0076] (1) Tablet core prescription:
[0077]
[0078]
[0079] (2) Prescription of semi-permeable membrane coating solution:
[0080]
[0081] (3) Preparation process: Weigh the components that have passed through a 100-mesh sieve according to the prescription and mix them evenly; finally add the prescribed amount of magnesium stearate, mix evenly, and press into tablets; Coating until the weight gain of the tablet core is 4%; use a laser to punch a small hole with a diameter of 0.8 mm on one side of the coated tablet.
[0082] (4) Measuring method: Paddle method is adopted, 900mL degassed distilled water is used as release medium, and the rotating speed is 75 revolutions per minute. The release degree at different time points was measured, and the osmotic pump tablets were taken out at different dissolution time points, sealed in a desiccator filled with a desiccant (silica gel) at room temperature, and placed for 48 hours. Acquire two-dimensional images with a 18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com