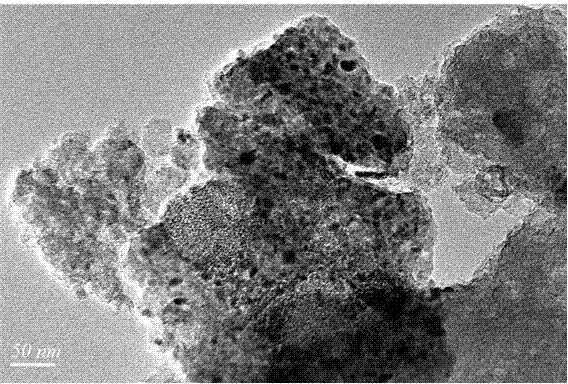
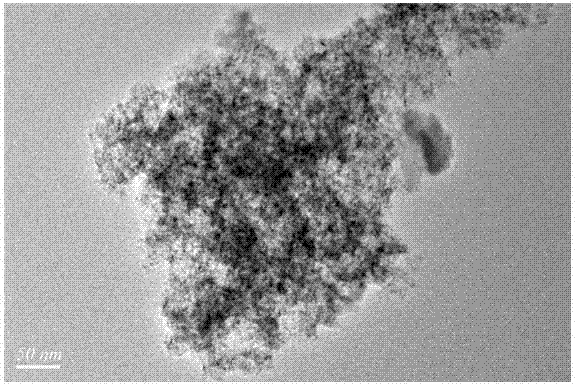
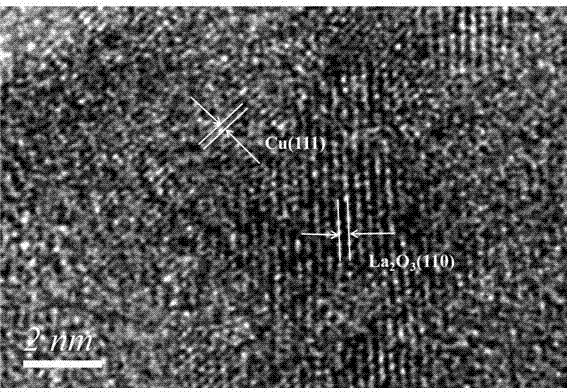
Chlorine-free bimetallic catalyst for gas phase synthesis of dimethyl carbonate and preparation and application
A technology of bimetallic catalyst and dimethyl carbonate, which is applied in the preparation of carbonate/haloformate, metal/metal oxide/metal hydroxide catalyst, and organic compound, can solve the problem of low catalytic activity, Chlorine loss, low product selectivity and other issues, to achieve good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Weigh 4.99 g of Cu(CH 3 COO) 2 ·H 2 O, measure 250 mL of deionized water, add it to a beaker, and stir it magnetically for 10 min to form a 0.1 mol / L copper acetate aqueous solution; weigh 2.16 g of La(NO 3 ) 3 ·6H 2 O, measure 250 mL of deionized water, add it to a beaker, and stir it magnetically for 10 min to form a 0.02 mol / L aqueous solution of lanthanum nitrate.
[0035] (2) Weigh 60-80 mesh coconut shell activated carbon (specific surface area 1600 m 2 / g, pore volume 0.8 cm 3 g -1 ) 10.0 g, added to the above mixed solution, placed in an ultrasonic reactor and stirred for 240 min;
[0036] (3) Place the beaker in a constant temperature water bath, evaporate the solvent to dryness at 80°C, and dry the evaporated product in an oven at 110°C for 8 hours;
[0037] (4) The dried precursor is roasted in a tube furnace with nitrogen flow, the nitrogen flow rate is 30ml / min, and the heating program is as follows: first increase to 280°C at a rate of 6°C / min, ...
Embodiment 2
[0044] (1) Weigh 12.08 g of Cu(NO 3 ) 2 ·3H 2 O, measure 250 mL of deionized water, add it to a beaker, and stir it magnetically for 10 min to form a 0.2 mol / L copper nitrate aqueous solution; weigh 0.91 g of La(CH 3 COO) 3 ·H 2 O, measure 250 mL of deionized water, add it to a beaker, and stir it magnetically for 10 min to form a 0.01 mol / L aqueous solution of lanthanum acetate.
[0045] (2) Weigh 80-100 mesh shell activated carbon (specific surface area 2400 m 2 / g, pore volume 0.8 cm 3 g -1 ) 10.0 g, added to the above mixed solution, placed in an ultrasonic reactor and stirred for 280 min;
[0046] (3) Place the beaker in a constant temperature water bath, evaporate the solvent to dryness at 70°C, and dry the evaporated product in an oven at 100°C for 15 hours;
[0047] (4) The dried precursor is roasted in a tube furnace with nitrogen flow, the nitrogen flow rate is 30ml / min, and the heating program is as follows: first increase to 300°C at a rate of 3°C / min, and ...
Embodiment 3
[0050] (1) Weigh 24.16 g of Cu(NO 3 ) 2 ·3H 2 O, measure 100 mL of deionized water, add it to a beaker, and stir it magnetically for 10 min to form a 1.0 mol / L copper nitrate aqueous solution; weigh 4.25 g of Ce(CH 3 COO) 3 ·6H 2 O, measure 100 mL of deionized water, add it to a beaker, and stir it magnetically for 10 min to form a 0.1 mol / L cerium acetate aqueous solution.
[0051] (2) Weigh 100-120 mesh coconut shell activated carbon (specific surface area 2800 m 2 / g, pore volume 0.6 cm 3 g -1 ) 18.0 g, added to the above mixed solution, placed in an ultrasonic reactor and stirred for 250 minutes;
[0052] (3) Place the beaker in a constant temperature water bath, evaporate the solvent at 60°C, and dry the evaporated product in an oven at 90°C for 16 hours;
[0053] (4) The dried precursor is roasted in a tube furnace with nitrogen flow, the nitrogen flow rate is 60ml / min, and the temperature rise program is as follows: first rise to 250°C at a speed of 8°C / min, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com