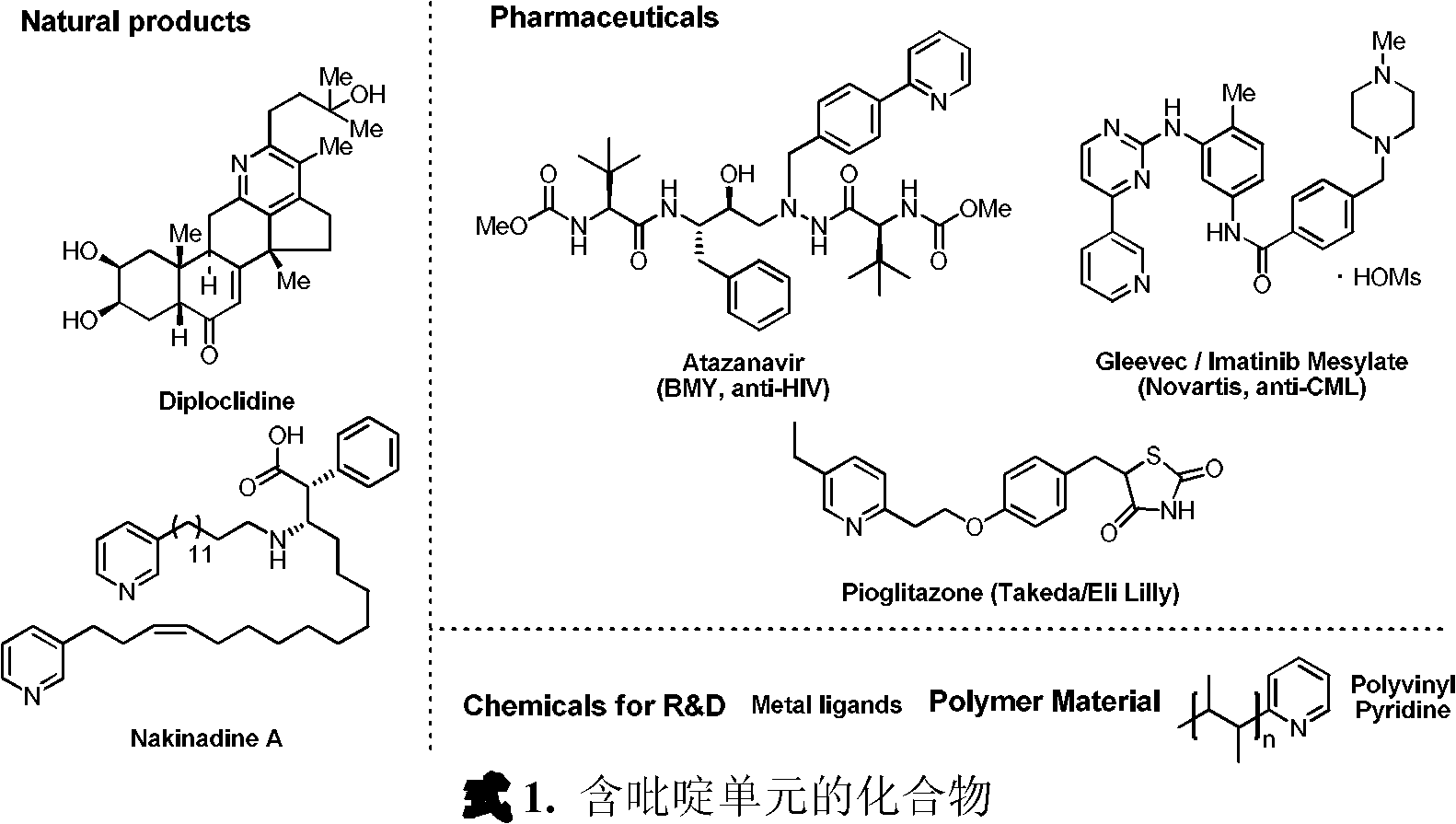
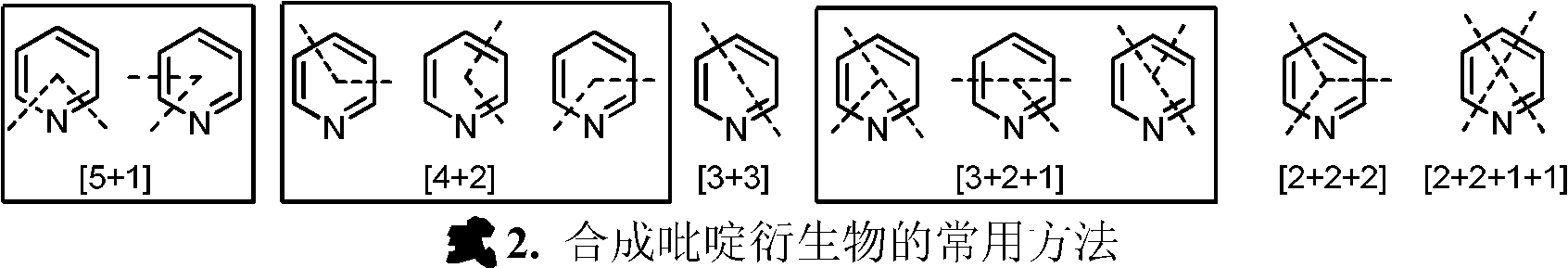
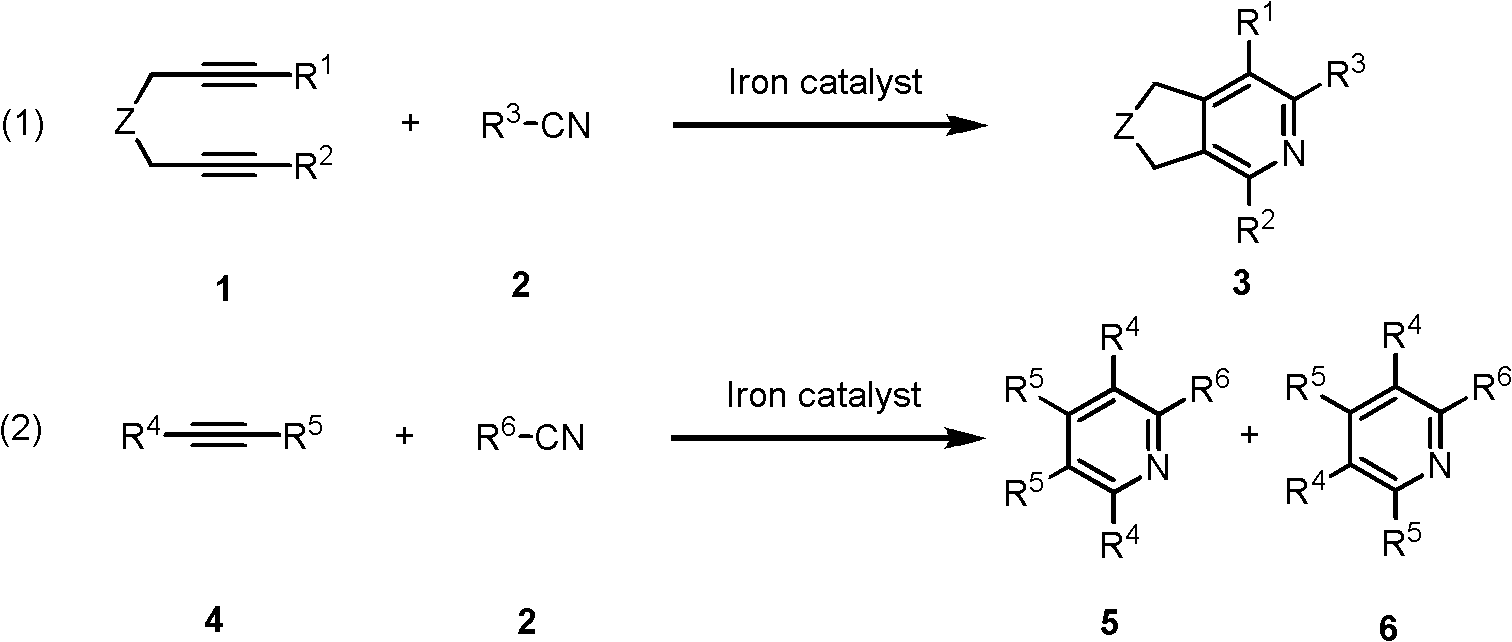
Iron catalyst system for preparation of pyridine derivatives and its application
A technology for iron catalysts and derivatives, which is applied in the field of iron catalysts for the preparation of pyridine derivatives, can solve the problems of slow development of catalytic systems, restrictions on the application of iron catalytic systems, and difficulty in preparation, and achieves simple and practical reaction operations, high yields, Easy to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Example 1: Preparation and screening of iron catalyst
[0021] Add iron metal precursor (0.05mmol) and bisphosphine ligand (0.10mmol) into the reaction flask, add 1mL tetrahydrofuran after argon replacement, and stir at room temperature for 0.5 hours. Then add reducing agent Zn (0.10mmol) and stir, then add diyne 1a (0.25mmol) and nitrile 2a (2.5mmol), react at room temperature for 24h. After the reaction, the solvent was removed, and the pure product was obtained by direct column chromatography separation, and its structure was confirmed by nuclear magnetic resonance. The reaction formula and ligand structure are shown in formula 4:
[0022]
[0023] Conversion rate and productive rate are determined (internal standard method) by gas chromatography, and part result is listed in Table 1:
[0024] Table 1. Screening of iron catalysts
[0025]
example 2
[0026] Example 2: Synthesis of pyridine derivatives by iron-catalyzed [2+2+2] cycloaddition of diynes and nitriles
[0027] Add FeI to the reaction flask 2 (0.05mmol) and dppp (0.10mmol), after argon replacement, 2mL tetrahydrofuran was added, and stirred at room temperature for 0.5 hours. Then the reducing agent Zn (0.10mmol), diyne 1 (0.5mmol) and nitrile 2 (2.5mmol) were added, and the reaction was stirred at room temperature for 24h. After the reaction, the solvent was removed, and the pure product 3 was obtained by direct column chromatography separation, and its structure was confirmed by nuclear magnetic resonance. The reaction formula and the yield of some products were as shown in formula 5:
[0028] The NMR data of some products are as follows:
[0029] 3a: 1 H NMR (400MHz, Acetone) δ7.57-7.30(m, 5H), 3.76(s, 6H), 3.62(s, 2H), 3.61(s, 2H), 2.40(s, 3H), 2.23(s, 3H); 13 C NMR (100MHz, Acetone) δ172.4, 157.2, 151.0, 150.3, 141.9, 133.3, 130.1, 128.6, 128.2, 124.8, ...
example 3
[0034] Example 3: Iron-catalyzed [2+2+2] cycloaddition reaction of monoalkynes and nitriles to synthesize pyridine derivatives
[0035] Add FeI to the reaction flask 2 (0.05mmol) and dppp (0.10mmol), after argon replacement, 2mL tetrahydrofuran was added, and stirred at room temperature for 0.5 hours. Then adding reducing agent Zn (0.10mmol), monoalkyne 4 (1mmol) and nitrile 2 (2.5mmol), the reaction was stirred at room temperature for 24h. After the reaction, the solvent was removed, and the pure products 5 and 6 were obtained by direct column chromatography separation, and its structure was confirmed by nuclear magnetic resonance. The reaction formula and the yield of some products were as shown in formula 6:
[0036]
[0037] The NMR data of some compounds are as follows:
[0038] 5: 1 H NMR (500MHz, Acetone) δ8.18 (dt, J=8.3, 1.7Hz, 2H), 7.83 (d, J=7.9Hz, 1H), 7.67 (d, J=8.0Hz, 1H), 7.53-7.47 (m, 4H), 7.47-7.40(m, 4H), 2.54(s, 3H); 13 C NMR (125MHz, Acetone) δ156.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com