Continuous flow method for preparing benzaldehyde from benzyl dichloride through hydrolysis
A technology of benzyl dichloride and benzaldehyde, which is applied in the field of chemical production, can solve the problems of high equipment requirements, unstable operation, and large amount of wastewater, and achieve the effects of improving efficiency, reducing equipment investment, and reducing wastewater discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
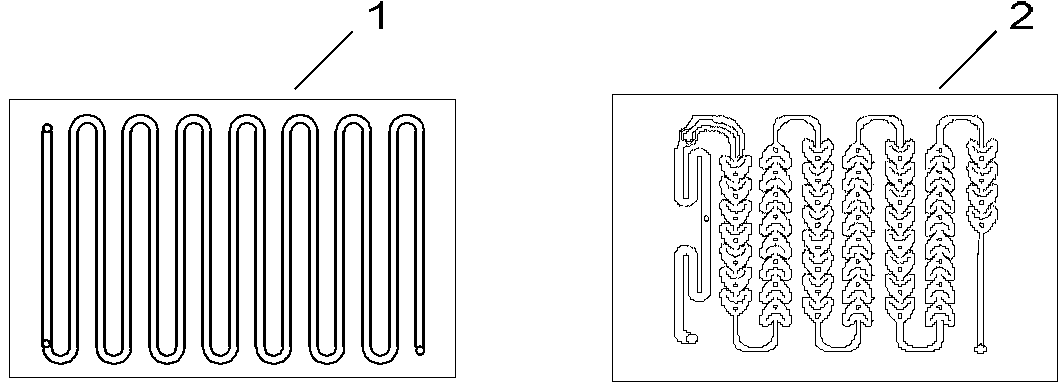
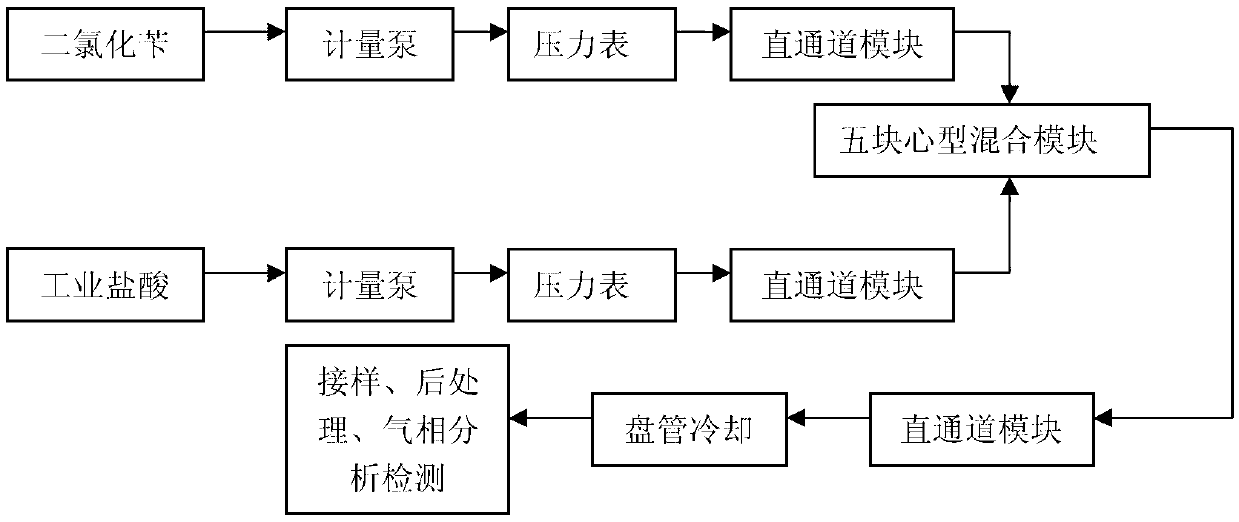
[0022] (1) The device used: select figure 1 Corning micro-channel reaction module (Corning straight channel module + Corning heart-shaped channel module), refer to figure 2 Determine the connection mode of the microchannel reactor, the number of mixed reaction modules is determined according to the flow rate and reaction residence time, and the heat transfer medium is heat transfer oil.
[0023] (2) Set the volume flow rate of each metering pump as F dichlorobenzyl = 10ml / min, F industrial hydrochloric acid = 15ml / min, put the materials into the straight channel glass module for preheating, and set the temperature of the heat exchanger At 120°C, the molar ratio of water to benzyl dichloride is 10:1. The residence time of the reaction was 115s. The reaction product flows out of the reactor in the state of continuous flow of oil-water mixed phase after being cooled by a coiled ice-water bath.
[0024] (3) The product can be detected by gas chromatography after oil-water sepa...
Embodiment 2
[0026] (1) The device used: select figure 1 Corning micro-channel reaction module (Corning straight channel module + Corning heart-shaped channel module), refer to figure 2 Determine the connection mode of the microchannel reactor, the number of mixed reaction modules is determined according to the flow rate and reaction residence time, and the heat transfer medium is heat transfer oil.
[0027] (2) Set the volume flow rate of each metering pump as F dichlorobenzyl = 10ml / min, F industrial hydrochloric acid = 15ml / min, put the materials into the straight channel glass module for preheating, and set the temperature of the heat exchanger At 130°C, the molar ratio of water to benzyl dichloride is 10:1. The residence time of the reaction was 115s. The reaction product flows out of the reactor in the state of continuous flow of oil-water mixed phase after being cooled by a coiled ice-water bath.
[0028] (3) The product can be detected by gas chromatography after oil-water sepa...
Embodiment 3
[0030] (1) The device used: select figure 1 Corning micro-channel reaction module (Corning straight channel module + Corning heart-shaped channel module), refer to figure 2 Determine the connection mode of the microchannel reactor, the number of mixed reaction modules is determined according to the flow rate and reaction residence time, and the heat transfer medium is heat transfer oil.
[0031] (2) Set the volume flow rate of each metering pump as F dichlorobenzyl = 10ml / min, F industrial hydrochloric acid = 15ml / min, put the materials into the straight channel glass module for preheating, and set the temperature of the heat exchanger At 140°C, the molar ratio of water to benzyl dichloride is 10:1. The residence time of the reaction was 115s. The reaction product flows out of the reactor in the state of continuous flow of oil-water mixed phase after being cooled by a coiled ice-water bath.
[0032] (3) The product can be detected by gas chromatography after oil-water sepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com