2-chloro-5-tirfluoromethylpyridine and synthetic method thereof
A technology of trifluoromethylpyridine and a synthesis method, applied in directions such as organic chemistry, can solve problems such as expensive catalyst manganese fluoride, ozone layer destruction by carbon tetrachloride, consumption of hydrofluoric acid, etc., and achieve low cost and production cost. Low, reduce the effect of intermediate links
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
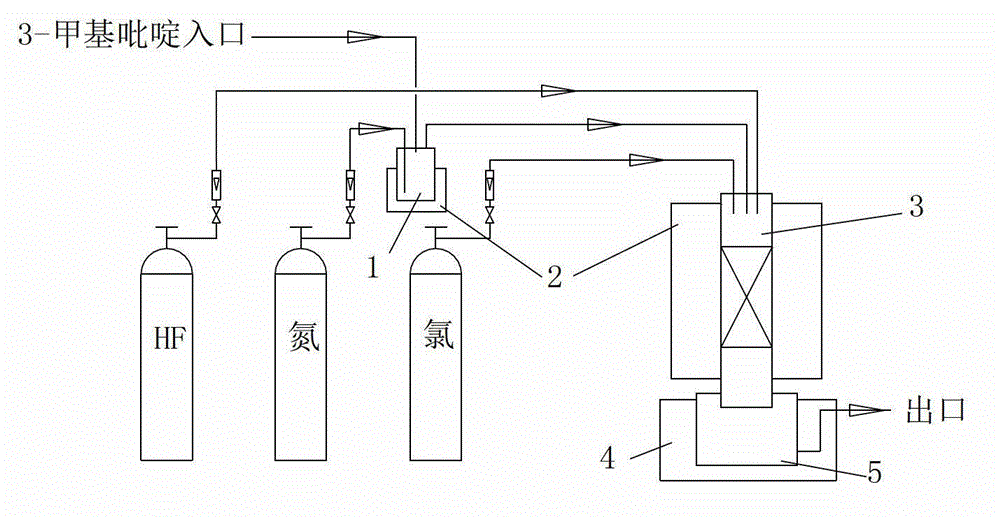
[0028] Such as figure 1 As shown, 2.5% CoCl by mass is charged in the reaction tube 3 2 / C 40g. After the temperature of reaction tube 3 rises to 380°C, chlorine gas and HF are introduced, wherein the chlorine gas flow rate is kept at 5 L / h, and the HF flow rate is kept at 10 L / h. Chlorine gas and HF are introduced for 1 hour (the purpose is to activate the catalyst). The vaporizer 1 and the reaction tube 3 are respectively heated by two heating furnaces 2 .
[0029] Pass 3-picoline into vaporizer 1 for vaporization, the temperature of vaporizer 1 is 250°C, and nitrogen gas is introduced at the same time to bring the vaporized 3-picoline into reaction tube 3 after the catalyst has been activated, where 3-methylpyridine The flow rate of pyridine is maintained at 5g / h, and the flow rate of nitrogen gas is maintained at 15L / h;
[0030] During the reaction process, 3-picoline, chlorine, HF and nitrogen maintain the original flow rate, and the tail gas leaving the reaction tube ...
Embodiment 2
[0033] Load the mass percent 2.8%CoCl in the reaction tube 2 / C 40g. After the temperature of the reaction tube rose to 380°C, chlorine gas and HF were introduced, wherein the chlorine gas flow rate was maintained at 5 L / h, and the HF flow rate was maintained at 10 L / h. Chlorine gas and HF gas were introduced for 1 hour (the purpose was to activate the catalyst).
[0034] Pass 3-picoline into the vaporizer to vaporize, the temperature of the vaporizer is 250°C, and at the same time pass nitrogen to bring the vaporized 3-picoline into the reaction tube after the catalyst has been activated, and the flow rate of 3-picoline Keep at 5g / h, nitrogen flow rate at 15L / h;
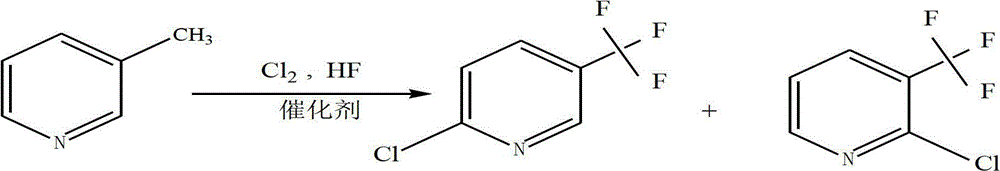
[0035] During the reaction process, 3-picoline, chlorine, HF and nitrogen maintain the original flow rate, and the tail gas leaving the reaction tube is condensed to obtain an oil, and the composition of the product is analyzed by conventional chromatographic methods. Among them, 2-chloro-5-trifluoromethylpyridine...
Embodiment 3
[0038] Load the mass percentage 3.5%CoCl in the reaction tube 2 / C 40g. After the temperature of the reaction tube rose to 380°C, chlorine gas and HF were introduced, and the chlorine gas flow rate was maintained at 5 L / h, and the HF flow rate was maintained at 10 L / h. Chlorine gas and HF gas were introduced for 1 hour (the purpose is to activate the catalyst).
[0039] Pass 3-picoline into the vaporizer to vaporize, the temperature of the vaporizer is 250°C, and at the same time pass nitrogen to bring the vaporized 3-picoline into the reaction tube after the catalyst has been activated, and the flow rate of 3-picoline Keep at 5g / h, nitrogen flow rate at 15L / h;
[0040] During the reaction process, 3-picoline, chlorine, HF and nitrogen were kept at the original flow rate, and the tail gas leaving the reaction tube was condensed to obtain an oil, and the composition of the product was analyzed by conventional chromatographic methods. Among them, 2-chloro-5-trifluoromethylpyri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com