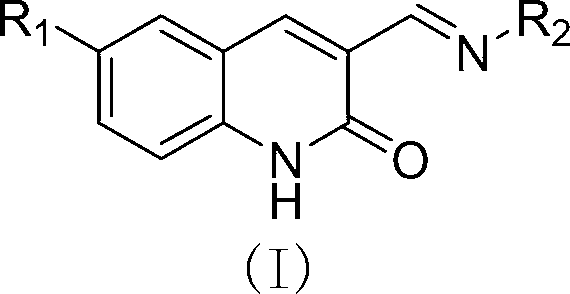
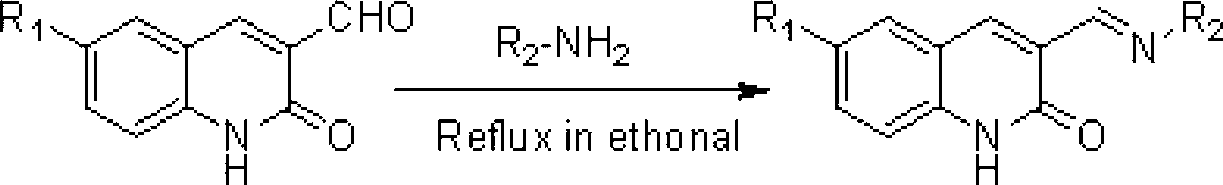3-Schiff base-2(1H)-quinolinone derivative and preparation method and application thereof
A quinolinone and Schiff base technology, applied in the field of medicine, can solve problems such as no public reports on quinolinone derivatives, and achieve the effects of improving electron mobility, short preparation period and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of 3-((n-butylimino)methyl)-2(1H)-quinolinone (compound 3)
[0028] Weigh 11.73g (1mmol) of the compound, 0.74g (1mmol) of n-butylamine, and 30mL of ethanol in a round-bottomed flask, and reflux at 85°C until the reaction is complete (TLC tracking detection, about 2h), after cooling, filter with suction. The filter residue was air-dried to obtain 1.89 g of compound 3 (light yellow solid), with a yield of 83%.
[0029] Compound 3 was analyzed, and its physicochemical and spectral properties were as follows:
[0030] Compound 3: Yields 83%, mp: 206~208°C; 1 H NMR (500MHz, DMSO-d 6 )δ12.04(s,1H,NH),8.54(s,1H,N=CH),8.43(s,1H,C=CH),7.82(d,J=7.7Hz,1H,Ar–H), 7.54(t,J=7.6Hz,1H,Ar–H),7.32(d,J=8.2Hz,1H,Ar–H),7.20(t,J=7.4Hz,1H,Ar–H),3.59( t,J=6.7Hz,2H,CH 2 ), 1.64–1.57 (m,2H,CH 2 ), 1.38–1.29 (m,2H,CH 2 ),0.91(t,J=7.4Hz,3H,CH 3 ); 13 C NMR (DMSO-d 6 ,125MHz)δ161.88,156.21,137.87,136.45,134.14,131.36,126.14,123.13,118.61,115.90,84.26,33.04,20.36,14....
Embodiment 2
[0033] Example 2: Preparation of 3-((n-butylimino)methyl)-6-methyl-2(1H)-quinolinone (compound 4)
[0034] Weigh 22.74g (2mmol) of compound, 1.48g (2mmol) of n-butylamine, and 50mL of ethanol in a round-bottomed flask, and react until complete at a temperature of 85°C and an ultrasonic frequency of 40kHz (TLC tracking detection, about 1h), suction filtered after cooling, and the filter residue was dried at 40° C. to obtain 3.79 g of compound 4 (light yellow solid), with a yield of 78%.
[0035] Compound 4 was analyzed, and its physicochemical and spectral properties were as follows:
[0036] Compound 4: Yields 78%, mp: 181–183°C; 1 H NMR (500MHz, DMSO-d 6 )δ11.81(s,1H,NH),8.53(s,1H,N=CH),8.34(s,1H,C=CH),7.59(s,1H,Ar–H),7.37(d,J =8.4Hz,1H,Ar–H),7.23(d,J=8.4Hz,1H,Ar–H),3.58(t,J=6.8Hz,2H,CH 2 ),2.33(s,3H,CH 3 ), 1.63–1.56 (m,2H,CH 2 ), 1.38–1.29 (m,2H,CH 2 ),0.91(t,J=7.4Hz,3H,CH 3 ); 13 C NMR (DMSO-d 6 ,125MHz)δ161.86,156.16,137.92,136.65,133.18,131.78,129.02,126.85,119.3...
Embodiment 3
[0039] Example 3: Preparation of 3-((benzylimino)methyl)-2(1H)-quinolinone (compound 5)
[0040] The method of Example 1 was repeated, except that benzylamine was used instead of n-butylamine to obtain 1.99 g of compound 5 (yellow solid).
[0041] Compound 5 was analyzed, and its physicochemical and spectral properties were as follows:
[0042] Compound 5: Yields 76%, mp: 181.3–185.8°C; 1 H NMR (500MHz, DMSO-d 6 )δ12.05(s,1H,NH),8.71(s,1H,N=CH),8.49(s,1H,C=CH),7.81(d,J=7.8Hz,1H,Ar–H), 7.54(t,J=7.7Hz,1H,Ar–H),7.38–7.31(5H,m,Ar–H),7.27(d,J=5.5Hz,1H,Ar–H),7.19(t,J =7.5Hz,1H,Ar-H),4.80(s,2H,CH 2 ); 13 C NMR (DMSO-d 6 ,125MHz)δ161.95,157.24,140.03,139.90,137.30,131.95,129.82,128.86,128.57,127.40,127.28,126.87,126.83,122.73,119.36,115.51,64.92. + .
[0043] Therefore, it can be determined that compound 5 is 3-((benzylimino)methyl)-2(1H)-quinolinone, and its structural formula is as follows:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com