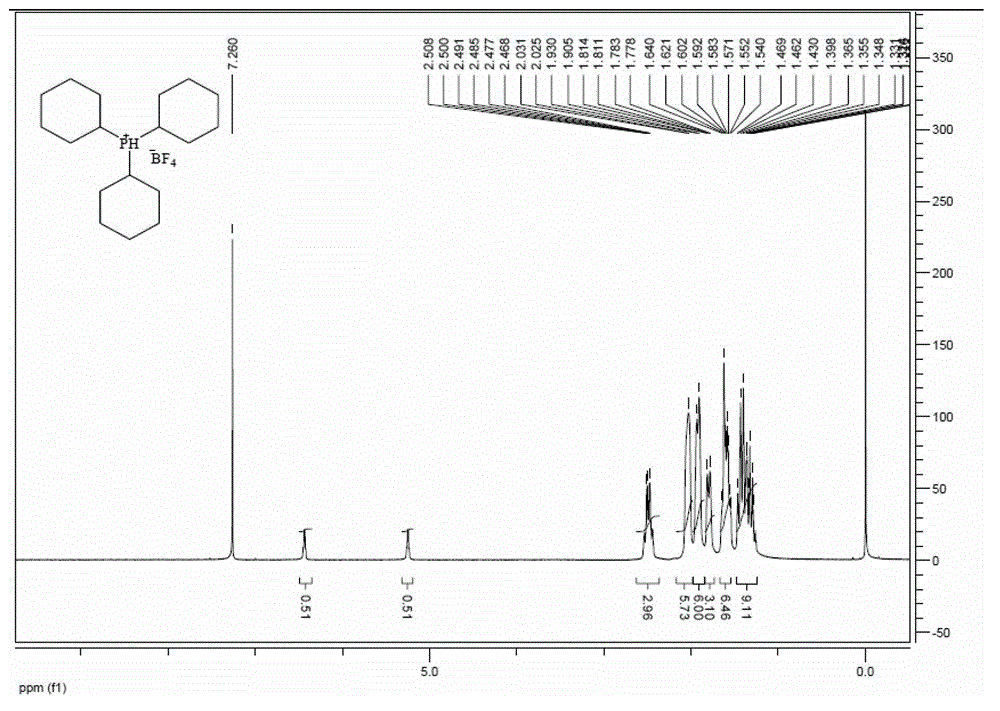
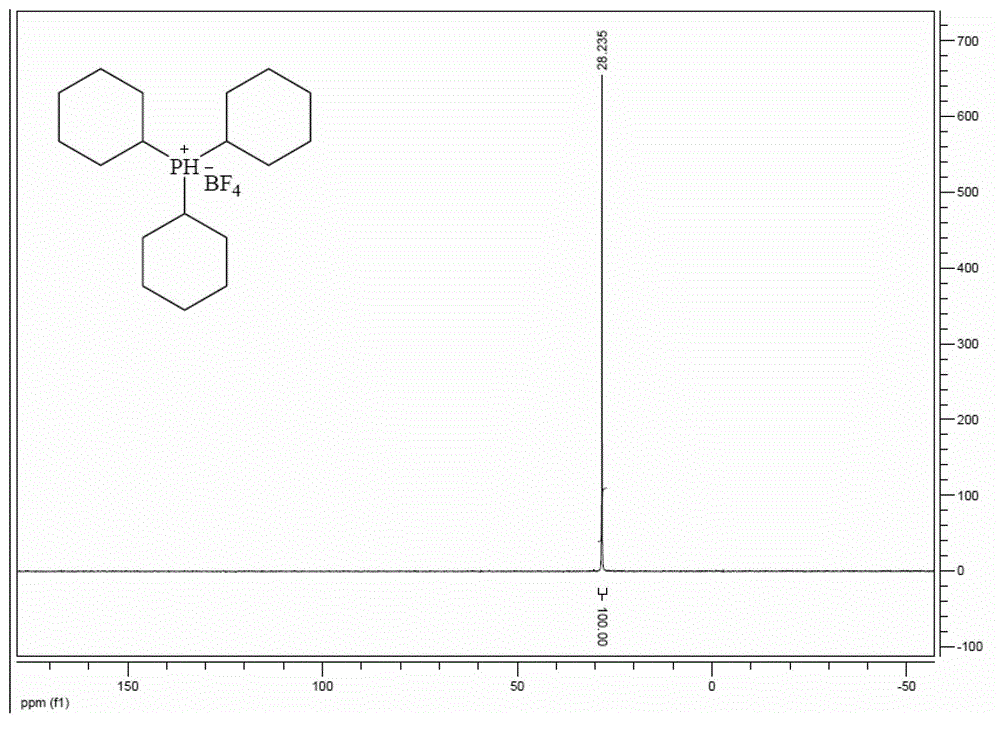
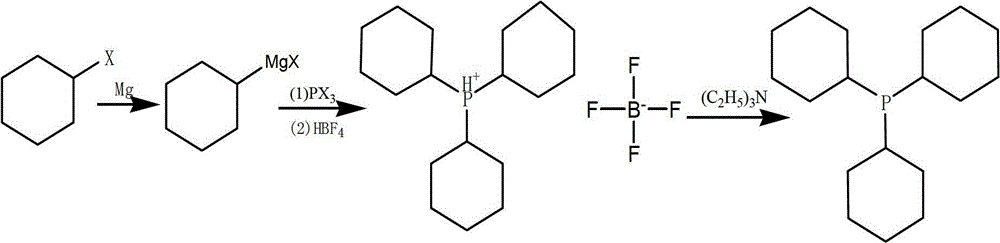
Preparation method of tricyclohexyl phosphine
A technology of tricyclohexylphosphine and cyclohexyl Grignard, which is applied in the field of preparation of tricyclohexylphosphine, can solve the problems of low product purity, low product yield, complicated processing and the like, and achieves simple post-processing, mild reaction conditions, Operational safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] 1. Preparation of Cyclohexyl Grignard Reagent
[0035] Under the protection of an inert gas such as nitrogen, magnesium chips and an organic solvent such as anhydrous tetrahydrofuran (THF) or anhydrous methyl tetrahydrofuran are added to the reaction vessel, the magnesium chips are slightly excessive, and the organic solvent can immerse the magnesium chips. Add a few grains of iodine, start the mechanical stirring, add the organic solvent solution of halocyclohexane such as anhydrous THF or anhydrous methyl tetrahydrofuran solution at 50-65°C, first add a small amount dropwise, after the Grignard reaction is triggered, then drop Add, the temperature is maintained at about 50-65 ° C, after the dropwise addition is completed, keep warm and stir. The reaction holding temperature is controlled at 30-70°C, preferably 45-65°C, and the holding time is controlled at 8-16 hours, preferably 12-16 hours.
[0036] 2. Preparation of Tricyclohexylphosphine
[0037] Lower the tempe...
Embodiment 1
[0048]Replace the reaction bottle with a nitrogen atmosphere, add 12.8g of analytically pure magnesium chips and anhydrous THF, and just immerse the magnesium chips in THF. Add 1-2 grains of iodine, start mechanical stirring, add 200mL of anhydrous THF solution of 59.3g of analytically pure chlorocyclohexane at 60°C, first drop 20mL and then stop the dropwise addition, after the Grignard reaction is triggered, then add dropwise , the temperature was maintained at 60°C, and after the dropwise addition was completed, it was stirred at 45°C for 12 hours.
[0049] The temperature of the reaction solution was lowered to 15°C, and 22g of analytically pure PCl was added dropwise 3 150 mL of anhydrous THF solution, the temperature was controlled at 25°C, and after the dropwise addition was completed, it was kept at 45°C for 1 hour.
[0050] After cooling down to room temperature, add saturated NH dropwise in an ice-water bath 4 Cl aqueous solution until the reaction liquid was clear...
Embodiment 2
[0057] Replace the reaction bottle with a nitrogen atmosphere, add 12.8g of analytically pure magnesium chips and anhydrous THF, and just immerse the magnesium chips in THF. Add 1-2 grains of iodine, start mechanical stirring, add 200mL of anhydrous THF solution of 59.3g of analytically pure chlorocyclohexane at 60°C, first drop 20mL and then stop the dropwise addition, after the Grignard reaction is triggered, then add dropwise , the temperature was maintained at 60°C, and after the dropwise addition was completed, it was stirred at 60°C for 16 hours.
[0058] The temperature of the reaction solution was lowered to 15°C, and 22g of analytically pure PCl was added dropwise 3 150 mL of anhydrous THF solution, the temperature was controlled at 25°C, and after the dropwise addition was completed, it was kept at 40°C for 2 hours.
[0059] After cooling down to room temperature, add saturated NH dropwise in an ice-water bath 4 Cl aqueous solution until the reaction liquid was cle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com