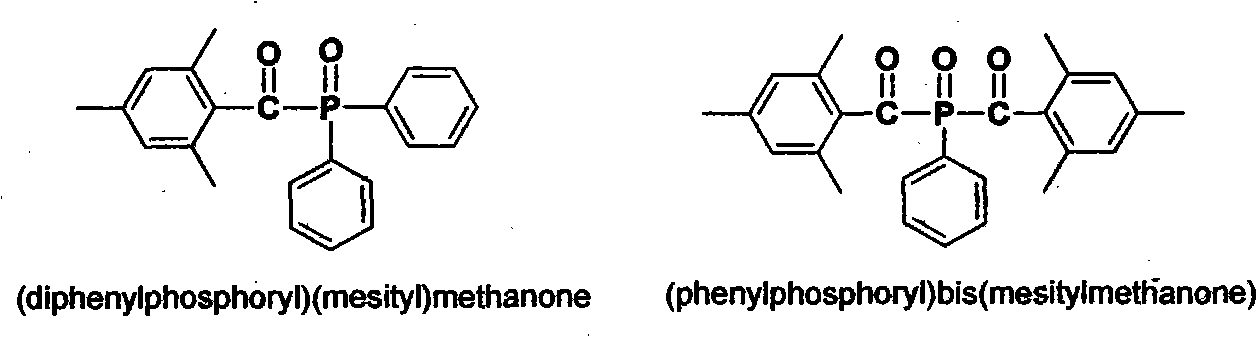
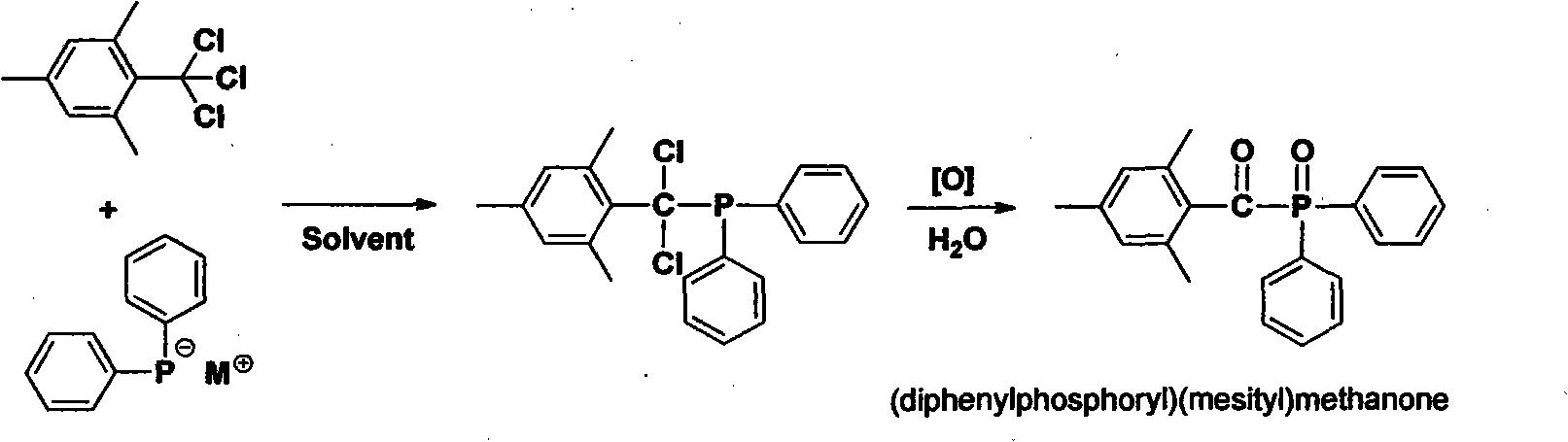
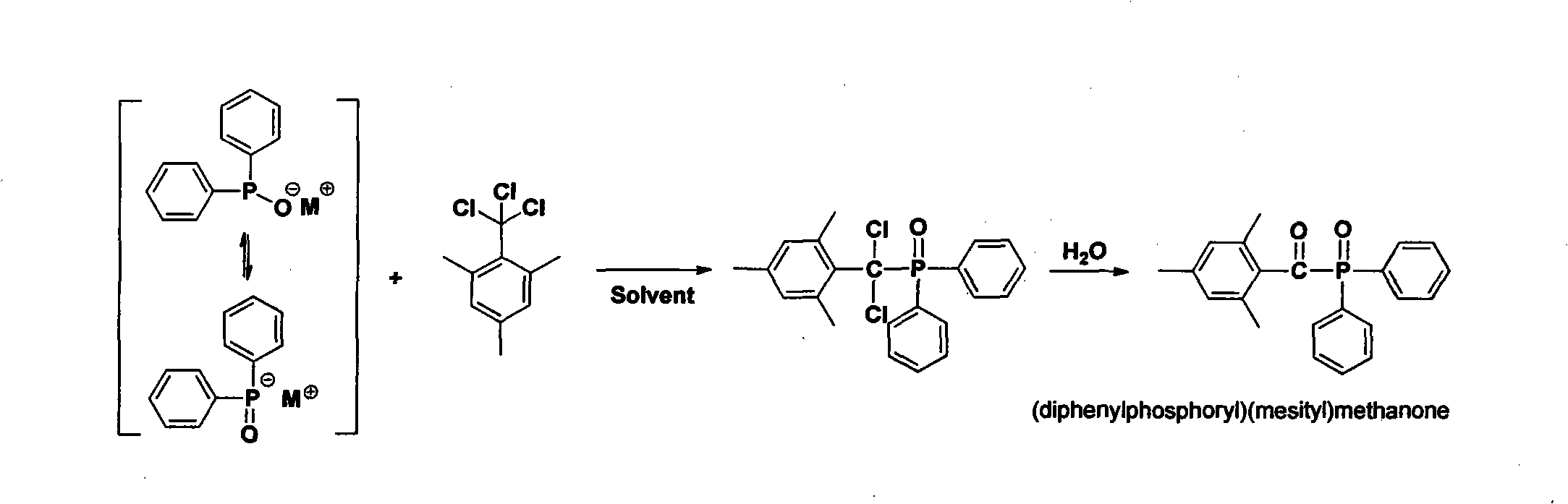
Preparation method of (diphenylphosphine oxide)(mesitylene)ketone and (phenylphosphine oxide)bis(mesitylene ketone)
A technology of diphenylphosphineoxy and mesitylene, which is applied in the field of new materials for free radical polymerization of light radiation, can solve the problems of difficult and complete reaction, large steric hindrance, and significant environmental risks, and achieve a significant effect of environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The technical process will be further described below in conjunction with specific examples.
[0019] The preparation of target compound (diphenylphosphinyl) (mesityl) ketone (route one):
[0020] Synthesis of 1,3,5-trimethyl-2-trichloromethyl-benzene: Mix 1.82 L of carbon tetrachloride and 2.5 kg of anhydrous AlCl in a reaction flask at room temperature 3 1.5 kg of mesitylene (i.e. 1,3,5-trimethylbenzene) was added dropwise within one hour under effective mechanical stirring, and the reaction system immediately appeared dark red and released hydrochloric acid gas (note the absorption of acid gas), and the addition was completed. Stirring was continued at 45 degrees Celsius for 2 hours, the reaction solution was slowly poured into an equal volume of 1N glacial hydrochloric acid, the organic phase was separated, extracted twice with an equal volume of dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, concentrated, and the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com