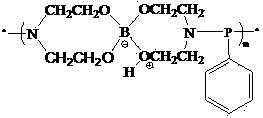
Polymer type phosphorus, nitrogen and boron containing flame retardant and preparation method thereof
A technology for polymers and flame retardants, applied in the field of chemical synthesis agents and their preparation, can solve the problems of poor polymer compatibility, great influence on flame retardant efficiency, influence on mechanical properties of materials, etc. Washable, environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 12.366g (0.2mol) of boric acid, 46.26g (0.44mol) of diethanolamine and 70ml of toluene into a three-necked flask equipped with magnetic stirring, thermometer, and oil-water separator, heat, stir and reflux until the separated water reaches 10.6ml to end the reaction. The toluene was distilled off under reduced pressure to obtain a light yellow transparent viscous liquid. Excess diethanolamine was removed by washing with tetrahydrofuran. Vacuum drying at 50°C gave 37.20 g of a colorless transparent viscous liquid with a yield of 85.30%. Its purity is 98.83% by titration with 0.1mol / L hydrochloric acid solution.
[0028] In the 250ml four-necked bottle equipped with magnetic stirring, dropping funnel and thermometer, add diethanolamine borate 32.7g and 150ml methylene chloride, add 26.9g phenyl phosphorus dichloride in the dropping funnel, use it Dilute with 20ml of dichloromethane. After the diethanolamine borate is completely dissolved, start to add phenylphosphor...
Embodiment 2
[0031] Wherein the synthetic method of intermediate diethanolamine borate is with example 1.
[0032] In a 250ml four-necked bottle equipped with magnetic stirring, dropping funnel and thermometer, add 21.8g of diethanolamine borate and 100ml of acetonitrile, add 17.9g of phenylphosphorous dichloride in the dropping funnel, and dissolve it with 10ml of acetonitrile Dilute, after the diethanolamine borate is completely dissolved, start to add phenylphosphorous dichloride dropwise, at this time, the temperature rises, and a white solid is formed, continue to react for 1 hour after the dropwise addition; then slowly raise the temperature to 100°C, Keep the reaction at 100°C for 5 hours, cool to room temperature, filter, wash with 100ml of dichloromethane, filter, and dry at 50°C to obtain a white powder product.
[0033] Add 3.6g of diethanolamine borate and phenylphosphorus dichloride co-condensation polymer, 1.2g of melamine, 35ml of DMF, and 5ml of pyridine into a three-necked...
Embodiment 3
[0035] Wherein the synthetic method of intermediate diethanolamine borate is with example 1.
[0036] In a 500ml four-necked bottle equipped with magnetic stirring, dropping funnel and thermometer, add 43.6g of diethanolamine borate and 200ml of acetonitrile, add 35.8g of phenylphosphine dichloride in the dropping funnel, and dissolve it with 20ml of acetonitrile Dilute, after the diethanolamine borate is completely dissolved, start to add phenylphosphorous dichloride dropwise, at this time, the temperature rises, and a white solid is formed, continue to react for 2 hours after the addition; then slowly raise the temperature to 85°C, Keep the reaction at 85°C for 12 hours, filter, take out the solid, wash with 100ml of dichloromethane, filter, and dry at 50°C to obtain a white powder product.
[0037] Add 10.8g of diethanolamine borate and phenylphosphorus dichloride co-condensation polymer, 2.82g of phenol, 80ml of DMF, and 4.250g of triethylamine into a three-necked flask wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com