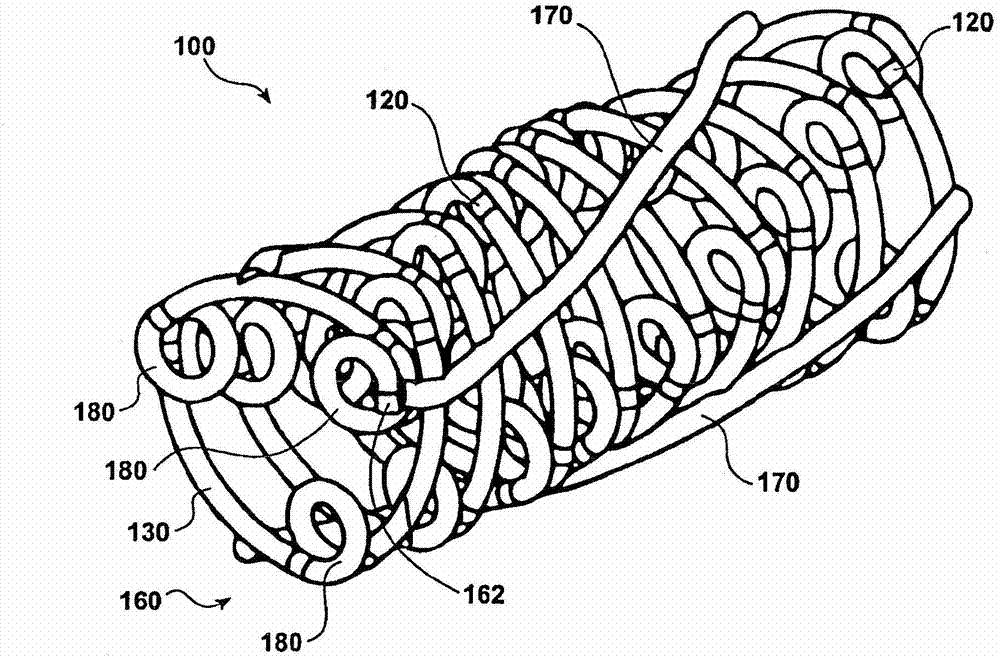
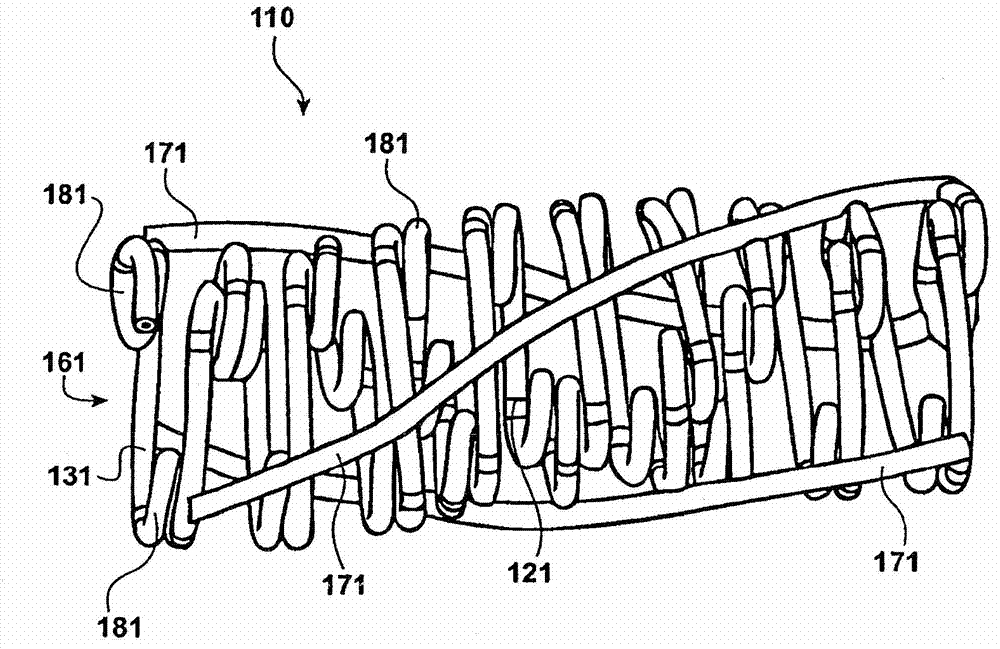
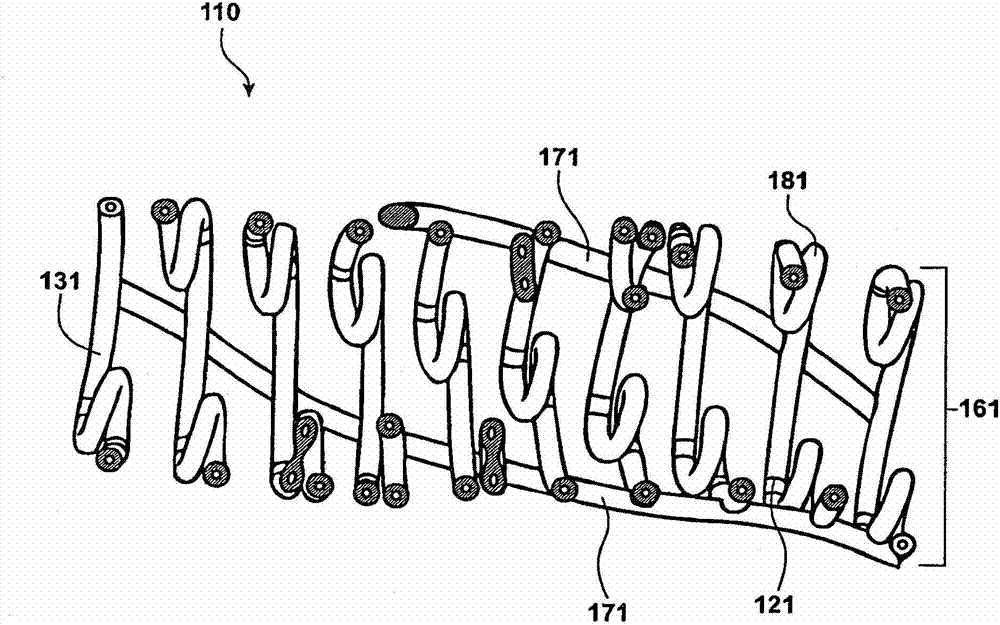
Temporal intraluminal stent, methods of making and using
A polymer tube, tubular structure technology, applied in stents, other medical devices, pharmaceutical sciences, etc., can solve the problem of lack of mechanical strength, elastic polymer recoil, etc., and achieve the effect of improving radiopacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Preparation of biodegradable polymer tubes
[0117] The biodegradable polymer tube was prepared by immersing the mandrel in a biodegradable polymer solution (12% by weight of PLLA in CCl 3 H (chloroform) solution) to form a coating layer by layer on a mandrel. The mandrel was dipped 46 times in the PLLA solution at a rate of about 0.1 m / s.
[0118] The dip-coated mandrel was then spin-dried around its longitudinal axis in a laminar flow hood to obtain a thin polymer layer after evaporation of the solvent. Repeat for spin drying. The final polymer tube was approximately 0.2mm thick and had 6% by weight phosphate buffer. The polymer coating was then solvent polished using chloroform or a pure solvent capable of dissolving the polymer and resulting in a thin and smooth polymer tube coating after drying.
[0119] The thickness of the outer diameter of the scaffold is reduced while maintaining the same inner diameter of the scaffold by stretching the tube scaffold from t...
Embodiment 2
[0123] Scaffold Design and Fabrication
[0124] Design the pattern of the scaffold using CAD software. Graphic designs and uncut biodegradable polymer tubing are sent to the laser lab for laser cutting. A variety of commercially available laser cutting equipment can be used, eg, Resonetics (Nashua, NH) and Spectralytics (Dassel, MN). Stent designs were cut from biodegradable polymer tube stents with an excimer laser at wavelengths less than 310 nm.
[0125] Those skilled in the art will appreciate that the stents of the invention disclosed in the disclosed embodiments or variations thereof have mechanical and therapeutic advantages over conventional stents. Furthermore, beneficial treatments would suggest that practitioners in the art employ them in view of the foregoing description of the invention. Due to the advantages of the biodegradable polymeric nature of the stent of the present invention, the same vessel site can be reprocessed at a later time if desired, including...
Embodiment 3
[0127] Radiopaque stents with iodine-containing contrast media
[0128] A PLLA polymer scaffold with a PLLA fiber diameter of 0.01905 cm and a fiber length of 15-22 cm and a length of 0.8-1.2 cm was used. Dissolve iohexol in methanol to a concentration of 350 mg / mL. Then, pure iohexol solution was sprayed on the top layer of the PLLA scaffold to a coating thickness of about 0.01″. The dose measured on all scaffold samples was 1000 μm / stent. When the methanol evaporated, the iohexol completely covered The surface of the near-chamber stent was exposed. After being exposed to water for 30 seconds, the radiopacity of the coated stent was observed under the C-arm (c-arm), and the results were as follows Figure 13 (#4). A control stent made of pure PLLA was also tested for radiopacity (#1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com