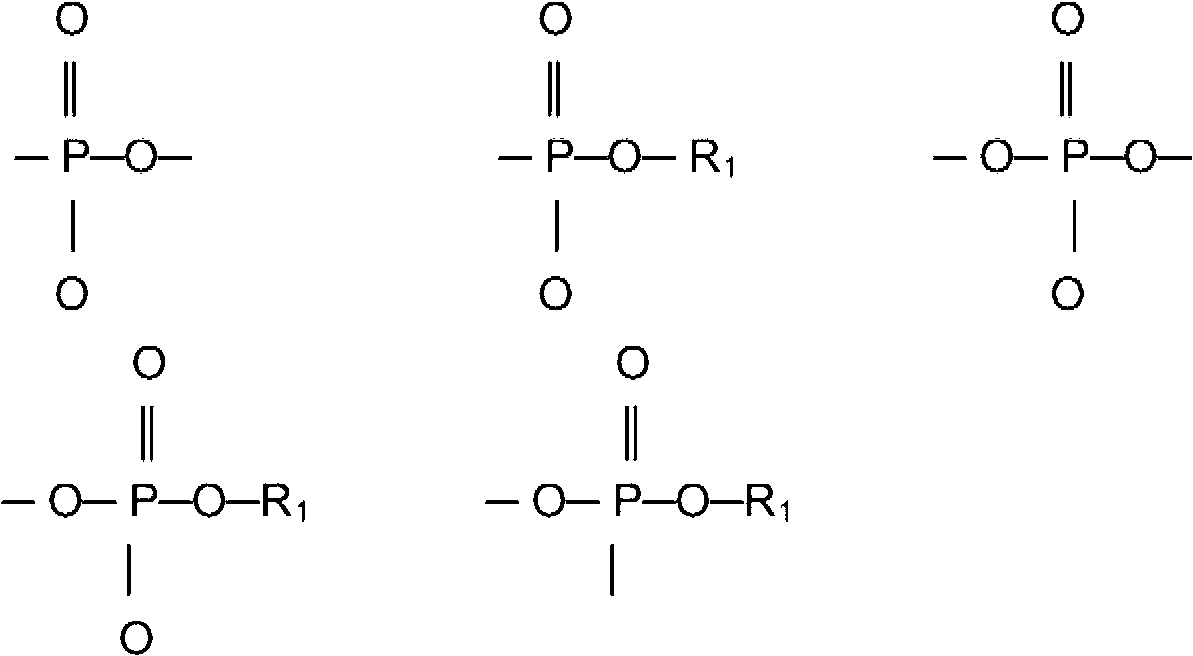
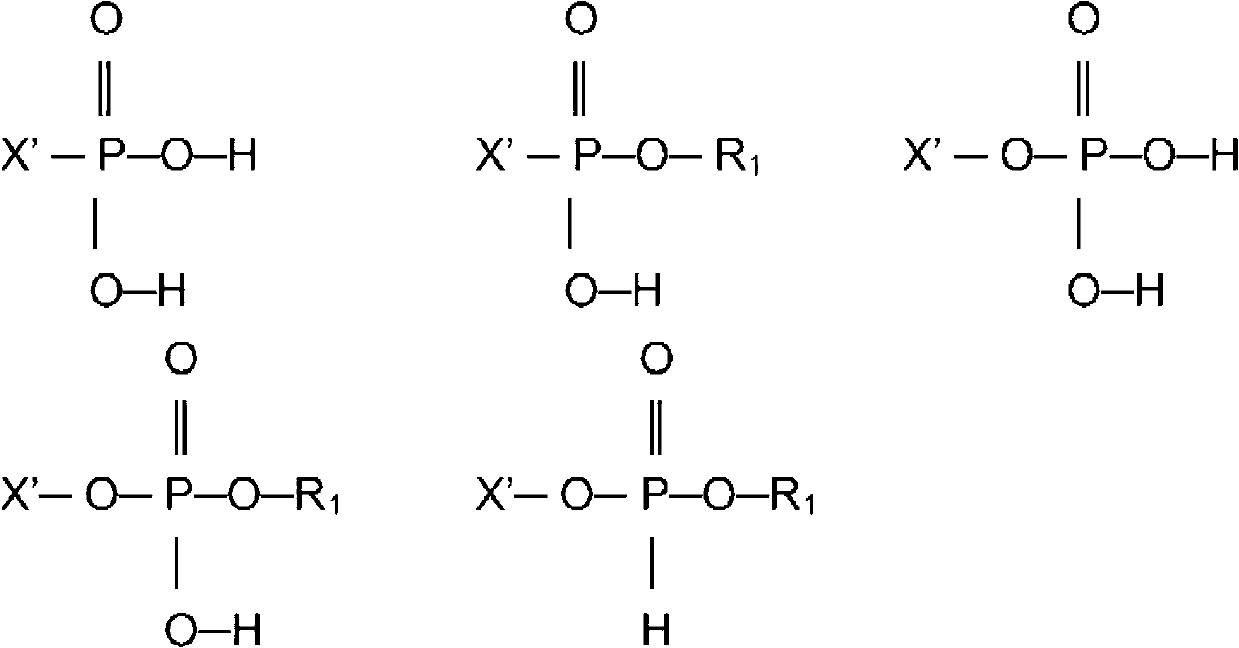
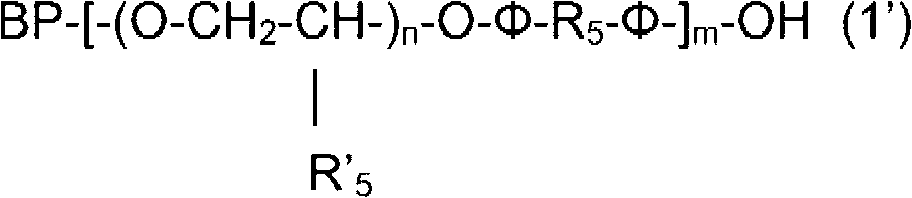Compounds having isocyanate functional group substituents and coating compositions comprised thereof
A cyanate-functional, isocyanate-based technology for metal substrates, paint or varnish type coatings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] This example relates to the preparation of compounds of the invention having isocyanate functionality and additionally comprising urethane functionality.
[0178] 450.5 g Tolonate HDT LV2, 48.5 g HBPA (0.203 mol) and 111.8 g n-butyl acetate were continuously fed into the reactor under nitrogen flow. The NCO of the starting Tolonate HDT LV2 was detected to be 0.537 mol / 100 g. The HBPA / (HBPA+starting isocyanate) ratio was 9.72%.
[0179] The mixture was heated at 120°C for 3 hours. NCO detection changed from 0.537 mol / 100 g at the start of Tolonate HDT LV2 to 0.331 of the reaction mixture at the end of the reaction.
[0180] 610.8 g of a formulation of the compound of the invention at 82% solids in n-butyl acetate were recovered.
[0181] The NCO detection of the preparation of the compound of the present invention is 0.33 mol / 100g. For a solids content of 82% in n-butyl acetate, its viscosity at 25° C. is 1305 mPa·s.
[0182] The product was separated by gel permeat...
Embodiment 2 and 3
[0186] These examples relate to the preparation of compounds of the invention having isocyanate functionality and additionally comprising urethane functionality.
[0187] The preparation was carried out as in Example 1, but the operating conditions and the HBPA / (HBPA+starting isocyanate) ratio were varied. The results are shown in Table 1 below.
[0188] Table 1
[0189]
[0190] Q1: Quantity of Tolonate HDT LV2
[0191] Q2: The amount of HBPA
[0192] R: HBPA / (HBPA+HDT LV2) weight ratio
[0193] T: reaction temperature; t: reaction time
[0194] ES: solid content
[0195] Ti: NCO detection, in mol / 100g
[0196] Ta: Residual HDI monomer level
Embodiment 4
[0198] This example relates to the preparation of compounds of the invention having isocyanate functionality and additionally comprising allophanate functionality.
[0199] 372.4 g of Tolonate HDT LV2 and 27.4 g of HBPA (0.114 mol) were continuously fed into the reactor under nitrogen flow. Dibutyltin dilaurate was added at 200 ppm relative to Tolonate HDT LV2. The NCO detection of the starting Tolonate HDT LV2 was 0.55 mol / 100 g. The HBPA / (HBPA+starting isocyanate) weight ratio was 6.85%.
[0200] The mixture was heated at 110°C for 48 hours. The NCO detection of the reaction mixture at the end of the reaction was 0.377 mol / 100 g.
[0201] 400 g of product were recovered.
[0202] The NCO assay of the formulation of the compound of the invention with allophanate functionality was 0.377 mol / 100 g. Its viscosity at 25°C and 90% solid content in n-butyl acetate is 14000mPa·s, and for 70% solid content in n-butyl acetate, its viscosity at 25°C is 800mPa·s.
[0203] The prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com