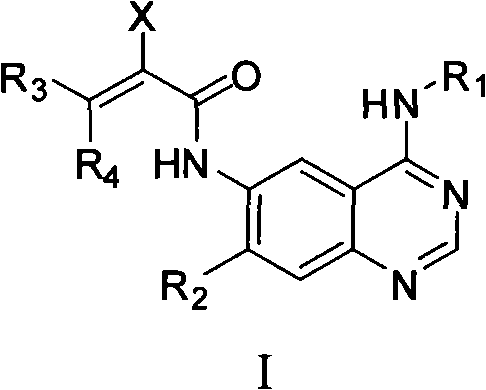
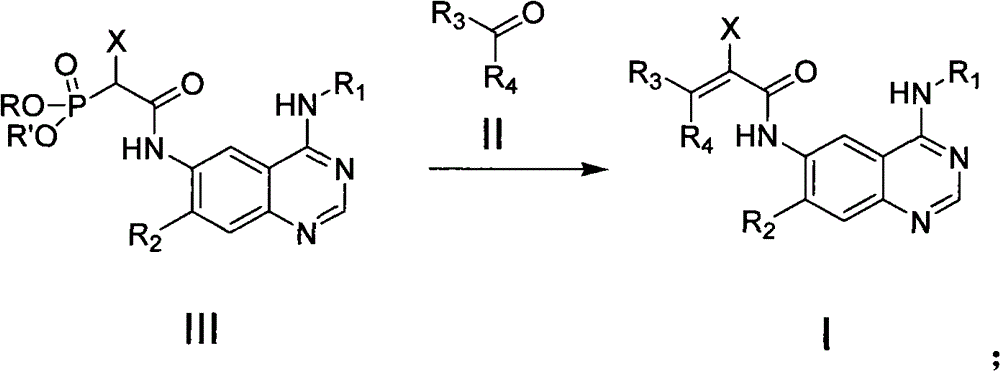
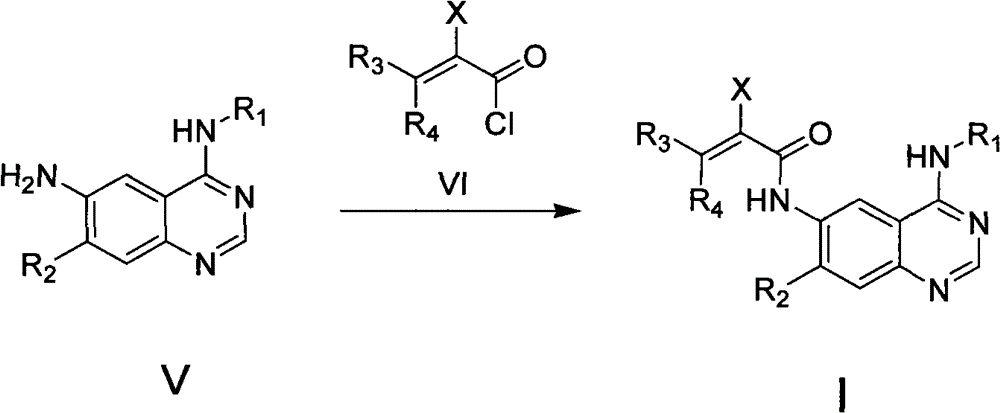
Quinazoline derivatives, preparation methods, intermediates, compositions and applications thereof
A technology of quinazoline and derivatives, which is applied in the field of quinazoline derivatives and can solve problems such as reversible inhibitor drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] N-(4-(3-chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-yl)-4-(dimethylamino)-2-fluorobut-2-enamide Preparation of (Compound 1)
[0198]
[0199] Step 1 Preparation of 4-(3-chloro-4-fluorophenylamino)-6-(2-fluoro-2-diethoxyphosphonoacetyl)amino-7-methoxyquinazoline
[0200] Raw material: 6-amino-4-(3-chloro-4-fluorophenylamino)-7-methoxyquinazoline was prepared according to the method in J.Med.Chem.2009, 52, 6880-6888.
[0201]Raw material: 2-fluoro-2-diethoxyphosphoryl acetyl chloride was prepared according to the method in the literature Heterocycles, 2004, 63, 699-706.
[0202] 6-Amino-4-(3-chloro-4-fluorophenylamino)-7-methoxyquinazoline (1 eq.) and triethylamine (1.5 eq.) were dissolved in DMF (10 ml), the The solution was stirred at 0 °C for 30 min. A solution of 2-fluoro-2-diethoxyphosphorylacetyl chloride (1.5eq.) in DMF (5ml) was slowly added dropwise to the above solution, and the reaction was stirred overnight at room temperature. After the reaction,...
Embodiment 2
[0211] (Z)-N-(4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-yl)-4-(dimethylamino)-2-fluorobutyl- 2-enamide and (E)-N-(4-(3-chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-yl)-4-(dimethylamino)- Preparation of 2-fluorobut-2-enamide
[0212]
[0213] The mixture of cis and trans isomers obtained in Example 1 was separated by using Gilson 215 semi-preparative chromatograph (322 type pump, 156 type UV detector).
[0214] Chromatographic column: Phenomenon Gimini 30×250mm, 10μm
[0215] Detection wavelength: 254nm
[0216] Column temperature: room temperature
[0217] Sample processing method: the sample (mixture of cis and trans isomers) was dissolved in methanol and obtained by filtration. The concentration is 22mg / ml, and the injection volume of each needle is 800μL.
[0218] Mobile phase: water: acetonitrile (with 0.05% ammonia added) = 49:51
[0219]
[0220] The fraction with a retention time of 14.5 min was collected to obtain the (Z)-type isomer (comp...
Embodiment 3
[0227] According to the same method as in Example 1, using different raw materials, the following compounds were prepared, all of which were mixtures of cis and trans isomers.
[0228] Compound 3-1: N-(4-(3-chloro-4-fluorophenylamino)-7-(2-methoxy)ethoxyquinazolin-6-yl)-4-(dimethyl Amino)-2-fluorobut-2-enamide
[0229]
[0230] The raw material 6-amino-4-(3-chloro-4-fluorophenylamino)-7-(2-methoxy)ethoxyquinazoline was prepared according to the method of document WO2008 / 33747; other raw materials were prepared as in Example 1 .
[0231] MS (ESI + ): m / z=492, 493, 494 [M+H] +
[0232] Rf value: 0.38 (silica gel, ethyl acetate / methanol=9:1)
[0233] Compound 3-2: N-(4-(3-chloro-4-fluorophenylamino)-7-ethoxyquinazolin-6-yl)-4-(dimethylamino)-2-fluorobutyl -2-enamide
[0234]
[0235] Raw material 6-amino-4-(3-chloro-4-fluorophenylamino)-7-ethoxyquinazoline according to 6-amino-4-(3-chloro-4-fluorophenyl in document WO2008 / 33747 Amino)-7-(2-methoxy)ethoxyquinazoline ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com