Anti-tumor and anti-bacterial dodecacyclo lactone compounds and use thereof
A technology of ester compounds and twelve-membered rings, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Twelve-membered ring lactone compounds 1 to 15 of the present invention ( dendrochliods A~O) Preparation
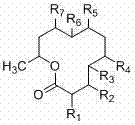
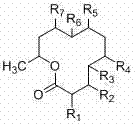
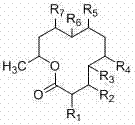
[0024] The present invention is from the symbiotic fungus of black milk sea cucumber in South China Sea Dendrodochium. 15 new twelve-membered ring lactone compounds dendrochliods A~O with antitumor and antibacterial activities isolated from sp., the above compounds have the following general chemical structure formula:
[0025]
[0026] The group collocation of described compound is as follows respectively:
[0027] .
[0028] Symbiotic fungi of sea cucumbers Dendrodochium. sp. (internal strain NO.10087) strain was isolated from the host black milk sea cucumber ( Holothuria nobilis Selenka). The strain was fermented in solid agar medium containing 5% biomalt extract (biomalt) for 28 days at room temperature, and then extracted several times with ethyl acetate. The extracts were combined, concentrated and evaporated to dryness under reduced p...
Embodiment 2
[0029] Example 2 Twelve-membered ring lactone compounds 1 to 15 of the present invention ( dendrochliods Identification of A~O)
[0030] 1. Dendrochliod A:C 12 h 20 o 5 , a colorless oil. 1 H and 13 See Table 1 for C NMR data.
[0031] 2. Dendrochliod B:C 12 h 18 o 5 , Pale yellow oil. 1 H and 13 See Table 2 for C NMR data.
[0032] 3. Dendrochliod C: C 12 h 18 o 5 , Pale yellow oil. 1 H and 13 See Table 3 for C NMR data.
[0033] 4. Dendrochliod D: C 12 h 18 o 5 , Pale yellow oil. 1 H and 13 See Table 4 for C NMR data.
[0034] 5. Dendrochliod E: C 13 h 20 o 5 , a colorless oil. 1 H and 13 See Table 5 for C NMR data.
[0035] 6. Dendrochliod F: C 13 h 20 o 5 , a colorless oil. 1 H and 13 See Table 6 for C NMR data.
[0036] 7. Dendrochliod G:C 12 h 20 o 6 , a colorless oil. 1 H and 13 See Table 7 for C NMR data.
[0037] 8. Dendrochliod H: C 12 h 18 o 4 , Pale yellow oil. 1 H and 13 See Table 8 for C NMR data.
[0038] 9. Dendr...
Embodiment 3
[0075] Example 3 Twelve-membered ring lactone compounds 1 to 15 of the present invention ( dendrochliods A~O) anti-tumor experiments
[0076] 1. Experimental method
[0077] The compound of the present invention was tested for inhibition of tumor cell proliferation by conventional MTT method.
[0078] 1. Cell lines used in the experiment: A549 (human lung cancer cells), Lovo (human colon cancer cells) and MG63 (human osteosarcoma cells). The cell lines used in the experiments were from the Institute of Cells, Chinese Academy of Sciences.
[0079] 2. Experimental reagents, consumables and instruments:
[0080] DMEM medium (Invitrigen); 1640 medium (Invitrigen); McCoy's 5a (Invitrigen); serum (Invitrigen); trypsin (Invitrigen); DMSO (sigma); MTT (sigma); Corning); pipette (Corning); 96-well plate (Corning); CO 2 Incubator (SANYO); microplate reader (Biotek76833)
[0081] 3. Experimental drugs:
[0082] Compounds 1-15 of the present invention (Dendrochliods A-O).
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com