Preparation method of toluene diisocyanate trimer curing agent
A technology of trimerization of toluene diisocyanate and isocyanate, applied in organic chemistry, polyurea/polyurethane coatings, coatings, etc., can solve the problems of low NCO content of curing agent, high viscosity, and difficulty in achieving high solid content, and achieve molecular weight Uniform distribution, reduced viscosity, rapid curing and anti-yellowing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
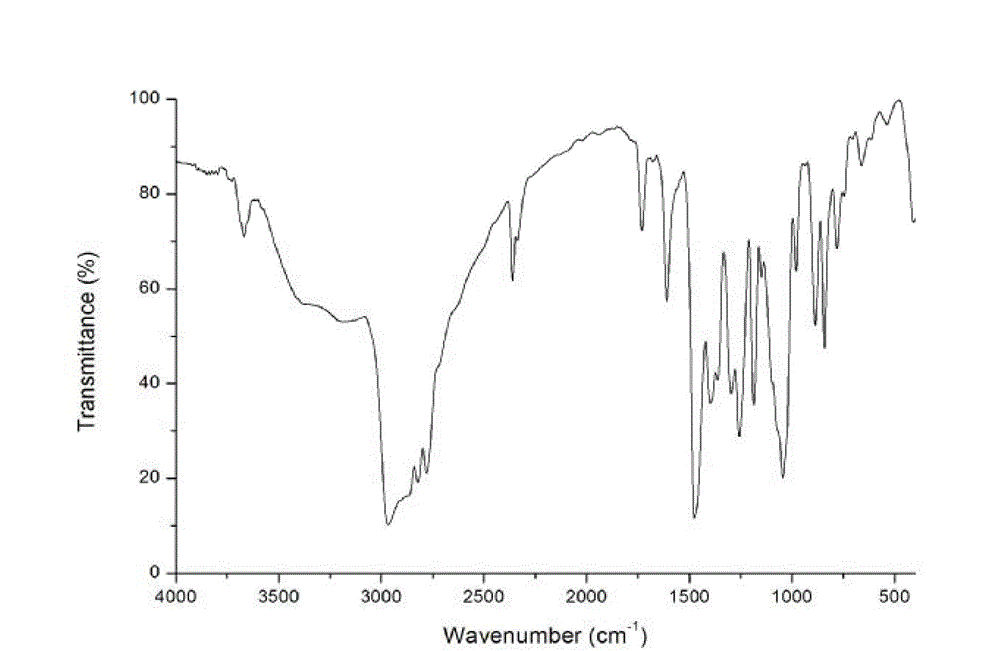
[0039] Take 18.8g of bisphenol A and 54.4g of dimethylamine solution, heat and stir at 50°C for 2h, add dropwise 46.5g of formaldehyde solution, raise the temperature to 80°C after dropping, keep the reaction for 2h, then stop heating. The reaction solution was layered, and the yellow organic phase was taken and distilled under reduced pressure at 130°C and -0.095MPa until no white solid was produced. The product was a yellow viscous liquid, which was dissolved in butyl acetate solvent to prepare a catalyst solution. figure 1 It is the infrared spectrogram of alkylaminomethylphenol catalysts, in which the broad peak phenol-OH stretching vibration at 3300~3100cm-1, aromatic hydrocarbon skeleton vibration at 1608cm-1, and phenol C-O stretching vibration at 1361cm-1 and 1256cm-1 prove There is a phenol structure in the product, and 885cm-1 and 841cm-1 are 1,2,4-trisubstituted and 1,2,3,5-tetrasubstituted benzene rings, indicating that one or two Substituents; while 1476cm-1 is th...
Embodiment 2
[0044] Take 18.8g of bisphenol A and 54.4g of dimethylamine solution, heat and stir at 50°C for 2h, add dropwise 46.5g of formaldehyde solution, raise the temperature to 80°C after dropping, keep the reaction for 2h, then stop heating. The reaction solution was layered, and the yellow organic phase was taken and distilled under reduced pressure at 130°C and -0.095MPa until no white solid was produced. The product was a yellow viscous liquid, which was dissolved in butyl acetate solvent to prepare a catalyst solution.
[0045] Pass N 2 Under protection, 100 g of TDI80 and 80 g of butyl acetate were put into the reaction vessel, and stirred at a constant temperature of 50° C. for 10 min. 11 g of a 5% homemade isocyanate trimerization catalyst butyl acetate solution containing alkylaminomethylphenols was added dropwise, and after the dropwise addition was completed, the mixture was stirred and reacted at 50° C. for 6 hours. The free NCO content was detected every 1 hour. When th...
Embodiment 3
[0047] Take 18.8g of bisphenol A and 54.4g of dimethylamine solution, heat and stir at 50°C for 2h, add dropwise 46.5g of formaldehyde solution, raise the temperature to 80°C after dropping, keep the reaction for 2h, then stop heating. The reaction solution was layered, and the yellow organic phase was taken and distilled under reduced pressure at 130°C and -0.095MPa until no white solid was produced. The product was a yellow viscous liquid, which was dissolved in butyl acetate solvent to prepare a catalyst solution.
[0048] Pass N 2 Under protection, 100 g of TDI80 and 80 g of butyl acetate were put into the reaction vessel, and stirred at a constant temperature of 40° C. for 10 min. 11 g of a 5% homemade isocyanate trimerization catalyst butyl acetate solution containing alkylaminomethylphenols was added dropwise, and after the dropwise addition was completed, the mixture was incubated and stirred at 40° C. for 8 hours. The free NCO content was detected every 1 hour. When ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com