Method for recovering cobalt and nickel from waste iron nickel cobalt alloy
An iron-nickel-cobalt alloy and metal technology, applied in the field of waste iron-nickel-cobalt alloy recycling, can solve problems such as cumbersome operations, and achieve the effect of streamlining processes and saving energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
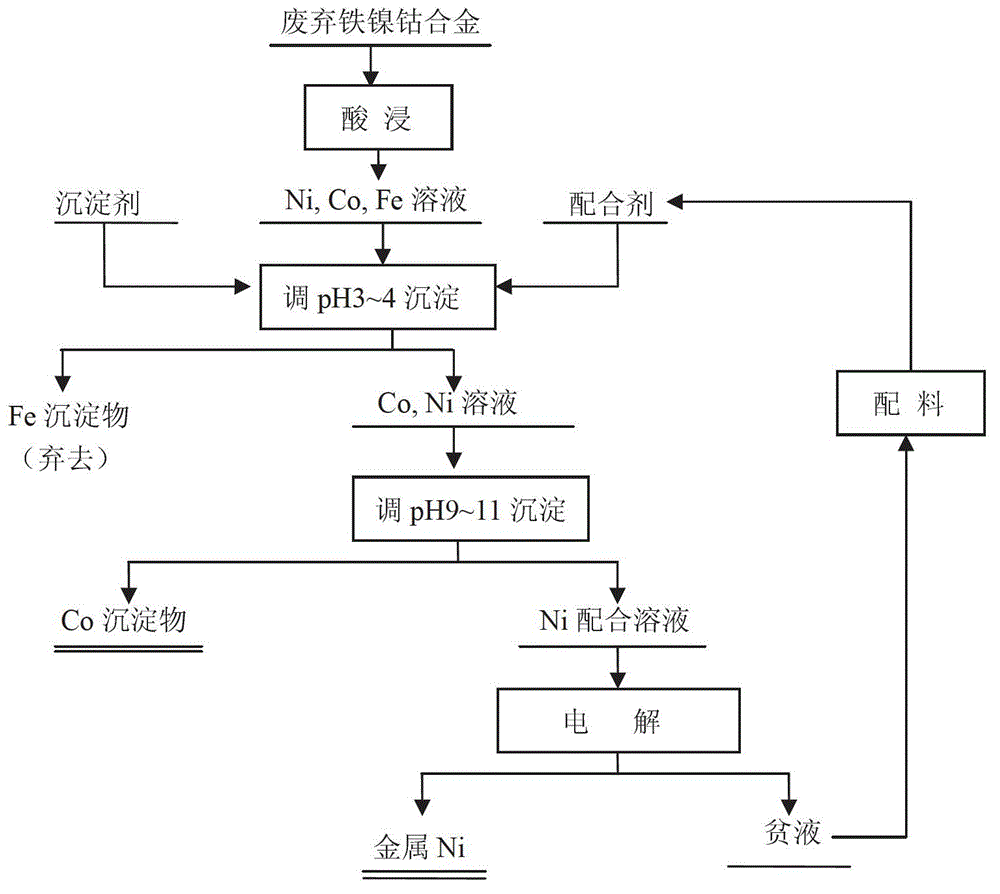
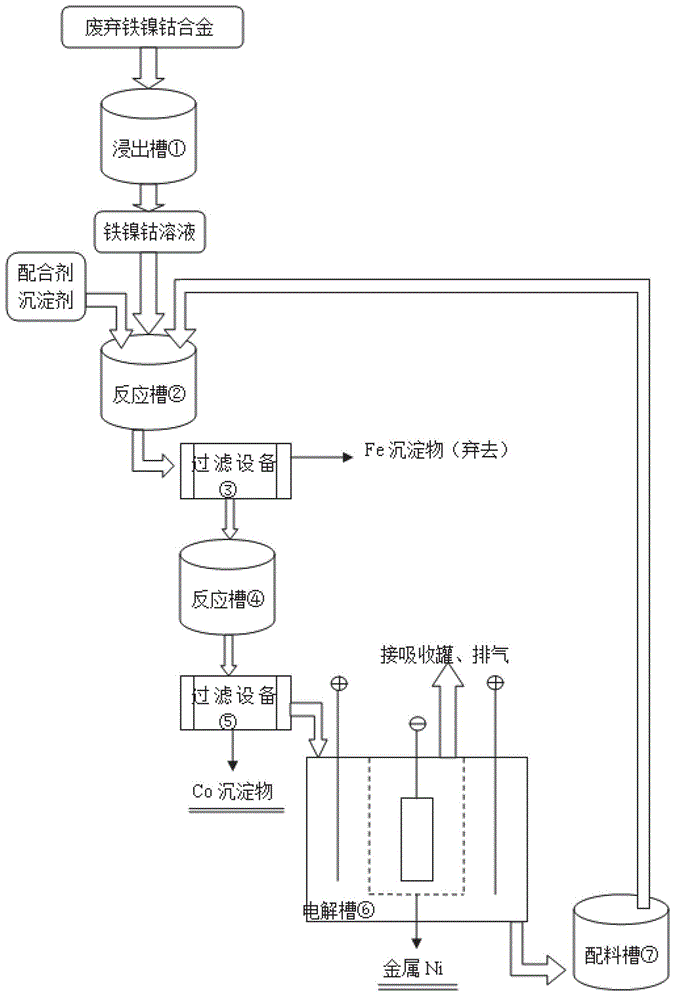
[0025] 1. According to figure 1 According to the process, take 5kg waste iron-nickel-cobalt alloy and put it into the leaching tank ① ( figure 2 shown), then add 50L of 2.5mol / L sulfuric acid to control the temperature at 65°C and stir for leaching, and add 2.5L of 30% hydrogen peroxide at the same time for leaching, and the leaching time is 4h.
[0026] 2. Put the waste iron-nickel-cobalt alloy leaching solution into the reaction tank ②, add 0.5L ammonia water and appropriate amount of NaOH, adjust the pH to 3, stir and react at room temperature for 1 hour, and obtain a solid-liquid mixture. The solid-liquid mixture is introduced into the filtration device ③ for filtration and separation, the obtained iron precipitate is discarded, and the filtrate is transferred to the reaction tank ④.
[0027] 3. Add 720g Na to the reaction tank ④ 2 CO 3 and 1.5 L of ammonia water, adjusted to pH = 10, and stirred at room temperature for 1 h to obtain a solid-liquid mixture. The solid-...
Embodiment 2
[0031] 1. According to figure 1 According to the process, take 5kg waste iron-nickel-cobalt alloy and put it into the leaching tank ① ( figure 2 shown), and then add 70L of 2.5mol / L sulfuric acid to control the temperature at 75°C and stir for leaching. At the same time, add 3.5L of 30% hydrogen peroxide, and the leaching time is 3h.
[0032] 2. Put the waste iron-nickel-cobalt alloy leaching solution into the reaction tank ②, add 0.5L ammonia water and appropriate amount of NaOH, adjust the pH to 3.5, stir and react at room temperature for 2 hours, and obtain a solid-liquid mixture. The solid-liquid mixture is introduced into the filtration device ③ for filtration and separation, the obtained iron precipitate is discarded, and the filtrate is transferred to the reaction tank ④.
[0033] 3. Add 1kg Na to the reaction tank ④ 2 CO 3 and 3.5 L of ammonia water, adjusted to pH = 11, and stirred at room temperature for 2 h to obtain a solid-liquid mixture. The solid-liquid mix...
Embodiment 3
[0037] 1. Take 5.5kg waste iron-nickel-cobalt alloy and put it into the leaching tank ① ( figure 2 shown), then add 100L of 2.5mol / L sulfuric acid to control the temperature at 85°C and stir for leaching, and add 5L of 30% hydrogen peroxide at the same time for leaching, and the leaching time is 2h.
[0038] 2. Put the waste iron-nickel-cobalt alloy leaching solution into the reaction tank ②, add an appropriate amount of NaOH, adjust the pH to 4, stir and react at room temperature for 3 hours, and obtain a solid-liquid mixture. The solid-liquid mixture is introduced into the filtration device ③ for filtration and separation, the obtained iron precipitate is discarded, and the filtrate is transferred to the reaction tank ④.
[0039] 3. Add 1.44kg Na to the reaction tank ④ 2 CO 3 And 6L Ethylenediamine C 2 N 2 h 8, adjust the pH=9, and stir the reaction at room temperature for 3h to obtain a solid-liquid mixture. The solid-liquid mixture is introduced into the filter devi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com