Method for producing tert-butyl ester compound by performing esterification reaction, rectification and coupling to organic carboxylic acid and isobutene
A reactive distillation and esterification reaction technology, applied in the chemical industry, can solve problems such as distillation pressure mismatch, and achieve the effects of low production cost, short process flow, and high economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
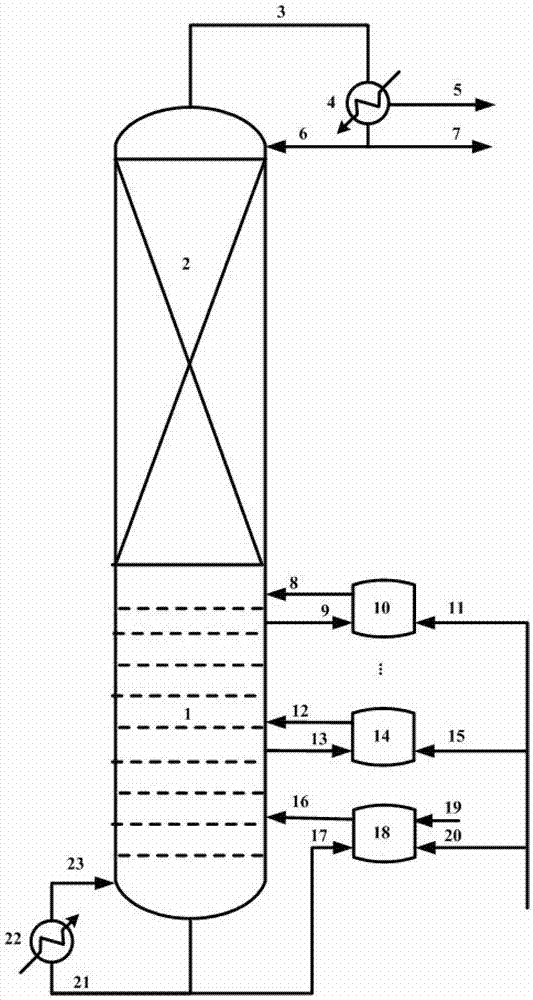
[0028] In a rectification column with an inner diameter of 1.0m and a tray number of 30 trays, two units with a volume of 5m 3 The tank reactor is connected, in which the material of the tower tank enters the first side reactor, and the outlet material of the side reactor enters the fifth tray (the number of trays is counted from bottom to top); from the sixth rectification tray The material enters the second reactor, the outlet material of the side reactor enters the 11th tray, and the material between the rectification column and the side reactor is transported by a pump.
[0029] Each side reactor is equipped with 150kg ion exchange resin catalyst (D006 type produced by Hebei Kairui Chemical Co., Ltd.). The feed flow rate of acetic acid to the first side reactor is 5 kmol / h, and the feed flow rate of isobutene to the first and second side reactors is 3.5 and 1.5 kmol / h, respectively. The esterification reaction temperature is 35°C, the pressure of each side reactor is 0.15...
Embodiment 2
[0031] In a rectification column with an inner diameter of 0.8m and a plate number of 30 trays, four units with a volume of 3m 3 The tank reactor is connected, in which the material in the tower tank enters the first side reactor, and the outlet material of the side reactor enters the first tray (the number of trays is counted from bottom to top); from the second rectification tray The material enters the second side reactor, and the material at the outlet of the side reactor enters the third tray; the material from the fourth rectification tray enters the third side reactor, and the outlet material of the side reactor enters the fifth column plate; the material from the 6th rectification tray enters the 4th side reactor, and the outlet material of the side reactor enters the 7th tray; the material between the rectification column and the side reactor is transported by a pump.
[0032]Each side reactor is equipped with 80kgY molecular sieve catalyst (Y molecular sieve produced...
Embodiment 3
[0034] In a rectification column with an inner diameter of 0.8m and a number of trays of 25 trays, three units with a volume of 3m 3 The tank reactor is connected, in which the material in the tower tank enters the first side reactor, and the outlet material of the side reactor enters the second tray (the number of trays is counted from bottom to top); from the third rectification tray The material enters the second side reactor, and the material at the outlet of the side reactor enters the fifth tray; the material from the sixth rectification tray enters the third side reactor, and the outlet material of the side reactor enters the eighth column The materials between the plates, rectification column and side reactors are pumped.
[0035] Each side reactor is equipped with 100kg HZSM-5 molecular sieve catalyst (the HZSM-5 molecular sieve produced by Nankai University Chemical Plant, with a silicon-aluminum ratio of 38). The feed flow rate of chloroacetic acid to the first sid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com