Kit for quantitatively detecting W515 site mutation of MPL genes
A site mutation and kit technology, applied in the field of fluorescent quantitative PCR, to achieve the effect of eliminating false positives and false negatives, good specificity, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1. Preparation of kit
[0038] 1. Design of specific primers and fluorescent probes
[0039]According to the gene sequence (ABL gene sequence and MPL gene sequence are from the National Center for Biotechnology Information Nucleic Acid Database, the ABL gene ID is 25, the reference sequence number is NM_005157.4; the MPL gene ID is 4352, the reference sequence number is NG_007525 .1) Design primers and fluorescent probes specific to the above-mentioned gene sequences respectively.
[0040] 2. Prepare the components of the kit according to the composition of the following kits
[0041] The kit of the present invention consists of the following:
[0042] ① Genomic DNA extraction reagent: Use tissue genomic DNA extraction kit (Qiagen Company, product number: 69504) to rapidly extract 0.5 ml of genomic DNA from the bone marrow tissue of patients with myeloproliferative neoplasms.
[0043] ② Primers, probes and standards: including MPL gene W515L site mutation-...
Embodiment 2
[0066] Embodiment 2. detect the MPL gene W515 site mutation with the kit prepared in embodiment 1
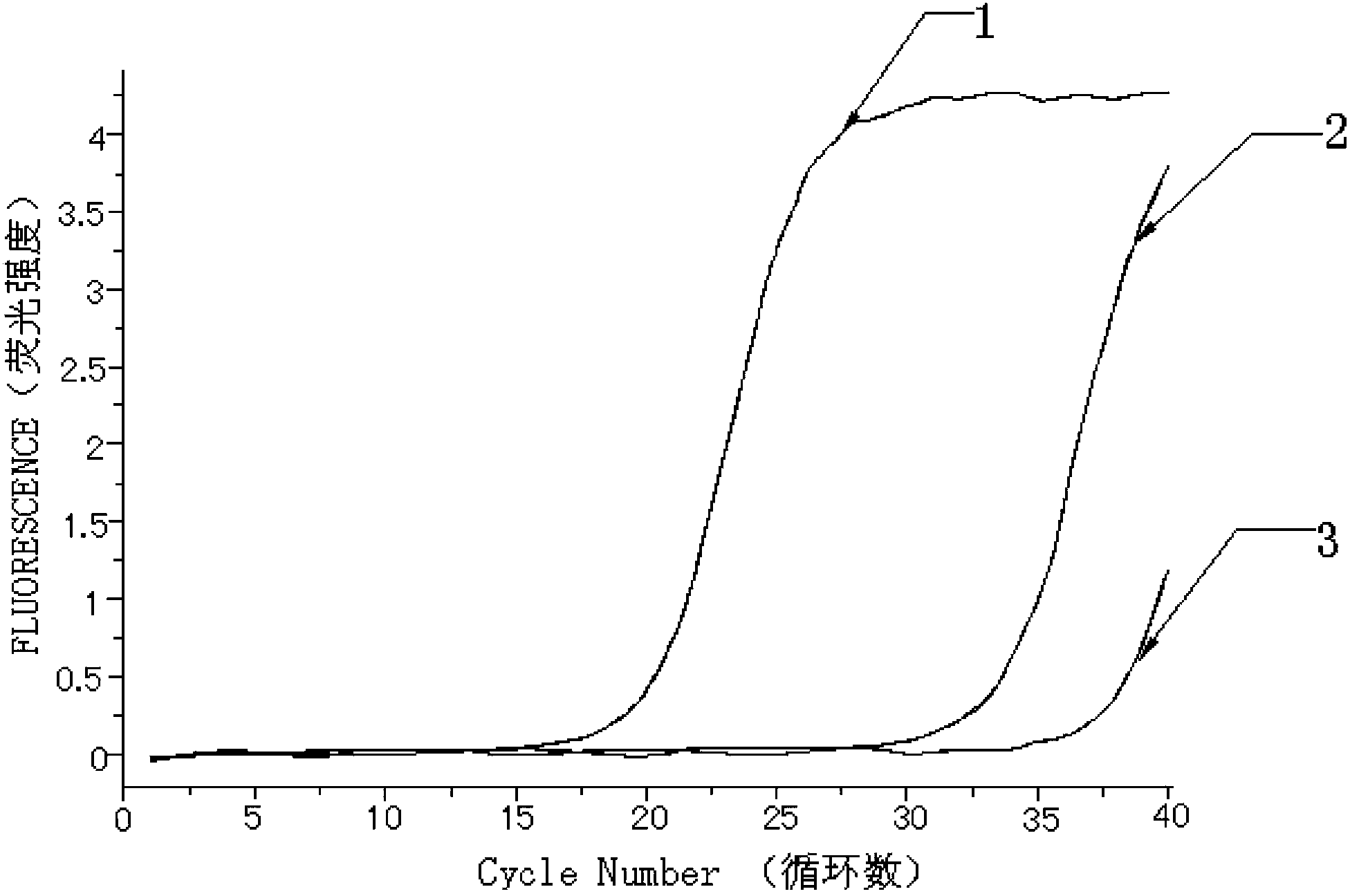
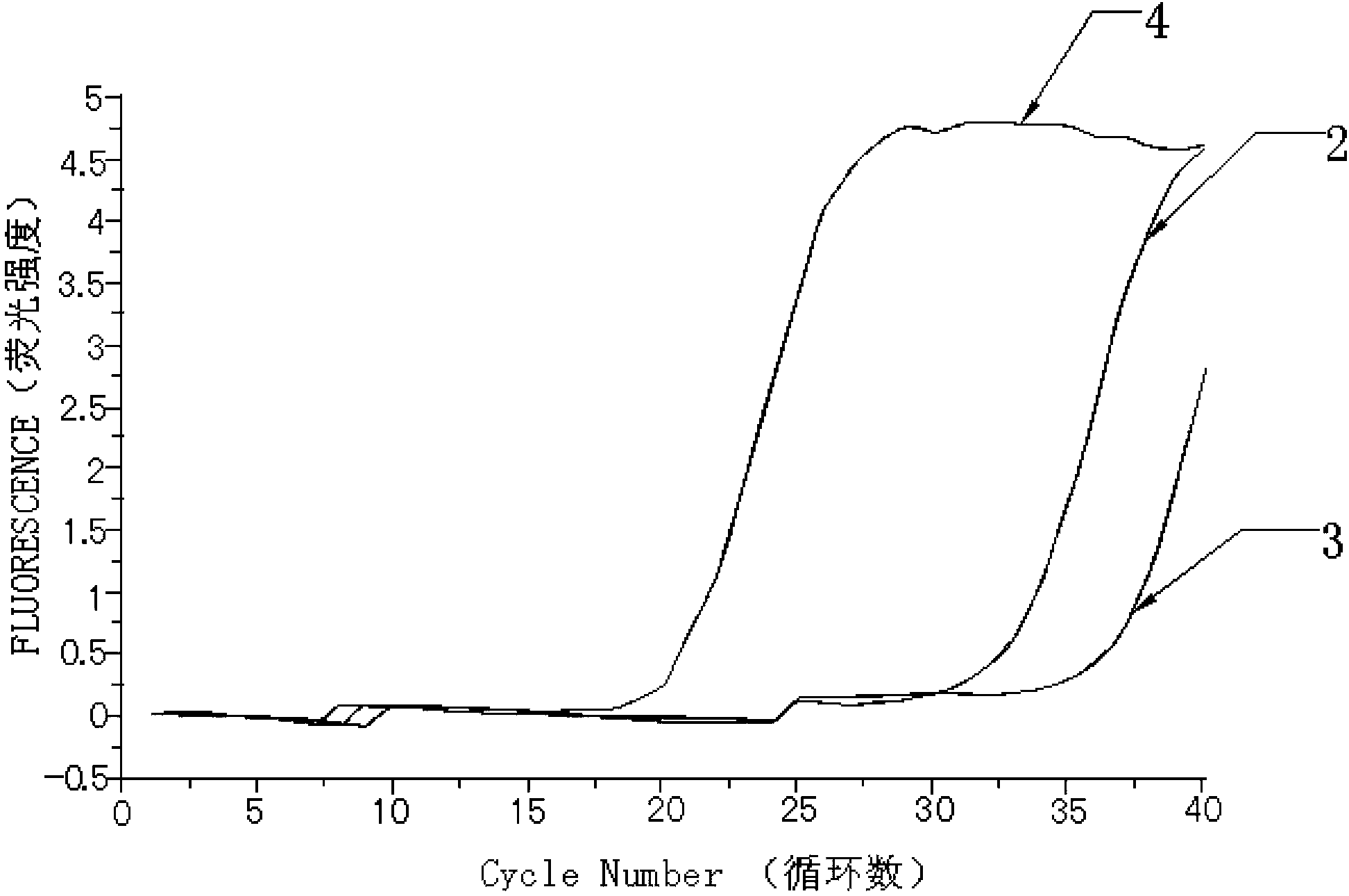
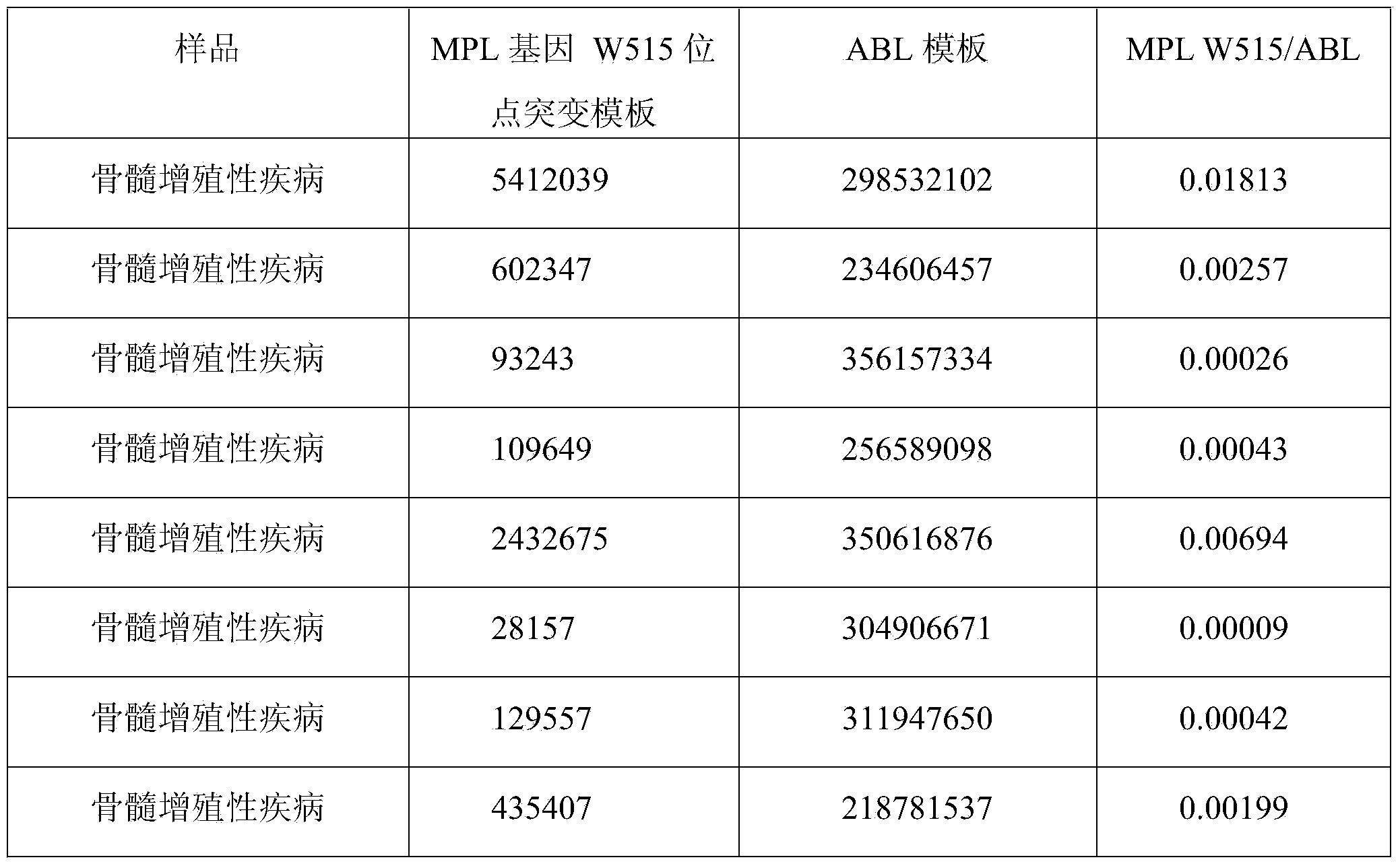
[0067] Take the detection results of bone marrow tissue samples from 30 patients with myeloproliferative neoplasms as an example.
[0068]The detection process of using the kit of the present invention to detect the mutation of the W515L site of the MPL gene and the mutation of the W515K site of the MPL gene is as follows: firstly, specific primers and fluorescent probes are designed according to the gene sequence. Secondly, obtain bone marrow tissue samples from patients with clinical myeloproliferative neoplasms, and quickly extract tissue DNA; first prepare the fluorescent quantitative PCR reaction solution of ABL internal reference gene and internal positive control sequence, and dilute the internal positive control sequence standard and ABL standard to the copy number respectively / mL is 1.0x10 3 , 1.0x10 4 , 1.0x10 5 and 1.0x10 6 , make the internal positive control se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com