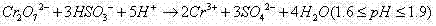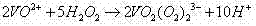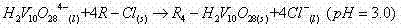Method for selectively separating and extracting vanadium and chromium from solution containing vanadium and chromium
A selective, solution-based technology, applied in the field of vanadium hydrometallurgy, can solve the problems of complex operation, serious environmental pollution, and high cost, and achieve the effect of simple equipment requirements, low process cost, and high-purity extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] 1.5L vanadium slag leaching solution containing vanadium (83mmol / L) and chromium (57mmol / L), after removing impurities by aluminum sulfate, at 50°C, the pH is based on the molar concentration of V(V) in the waste solution and 3 times of Cr(VI) Add 24g NaHSO to 0.6 times the sum of the molar concentrations 3 , heated and stirred at 65°C for 3h to reduce the vanadium and chromium in the mixed solution. then press H 2 o 2 / V molar ratio is 25 and 30wt% H is added 2 o 2The volume is 240ml, and then heated at 99°C until no more bubbles are generated, and the starch potassium iodide test paper is used to check that it does not turn blue. At this time, vanadium exists in the solution in the form of anion groups, and chromium exists in the form of Cr 3+ form exists.
[0039] Adjust the pH of the solution containing high-valent vanadium and low-valent chromium to 3.3, and the macroporous weakly basic chloride-type anion exchange resin in the exchange column selectively ads...
example 2
[0042] 1.5L vanadium slag leaching solution containing vanadium (83mmol / L) and chromium (57mmol / L), after removing impurities by aluminum sulfate, at 40°C, the pH is based on the molar concentration of V(V) in the waste solution and 3 times of Cr(VI) Add 28g NaHSO to 0.7 times the sum of the molar concentrations 3 , heated and stirred at 65°C for 3h to reduce the vanadium and chromium in the mixed solution. then press H 2 o 2 / V molar ratio is 30 and 30wt% H is added 2 o 2 The volume is 290ml, and then heated at 99°C until no more bubbles are generated, and the starch potassium iodide test paper is used to check that it does not turn blue. At this time, vanadium exists in the solution in the form of anion groups, and chromium exists in the form of Cr 3+ form exists.
[0043] Adjust the pH of the solution containing high-valent vanadium and low-valent chromium to 3.0, and the macroporous weakly basic chloride-type anion exchange resin in the exchange column selectively ad...
example 3
[0046] 1.5L vanadium slag leaching solution containing vanadium (83mmol / L) and chromium (57mmol / L), after removing impurities by aluminum sulfate, at 60°C, the pH is based on the molar concentration of V(V) in the waste solution and 3 times of Cr(VI) Add 32g NaHSO to 0.8 times the sum of the molar concentrations 3 , heated and stirred at 65°C for 3h to reduce the vanadium and chromium in the mixed solution. then press H 2 o 2 / V molar ratio is 25 and 30wt% H is added 2 o 2 The volume is 240ml, and then heated at 99°C until no more bubbles are generated, and the starch potassium iodide test paper is used to check that it does not turn blue. At this time, vanadium exists in the solution in the form of anion groups, and chromium exists in the form of Cr 3+ form exists.
[0047] Adjust the pH of the solution containing high-valent vanadium and low-valent chromium to 3.8, and the macroporous weakly basic chloride-type anion exchange resin in the exchange column selectively ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com