Pharmaceutical composition and use thereof in manufacturing medicaments for treating respiratory diseases
A technology for respiratory diseases and compositions, applied in the field of active compositions, can solve the problems of not being able to stop the decline of lung function, not significantly affecting the pathogenic mechanism, and contradictory clinical research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: Production of non-anticoagulant heparin
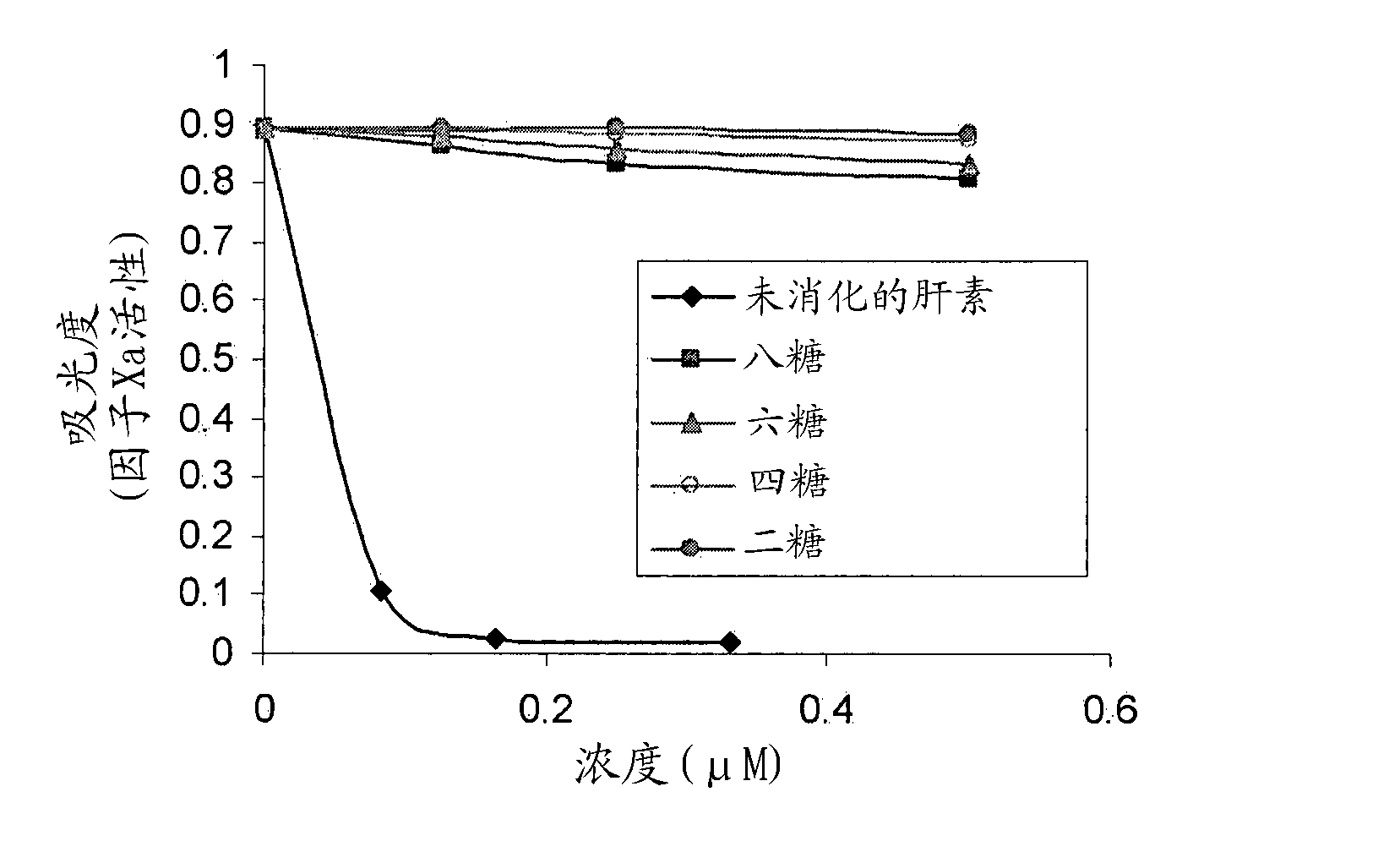
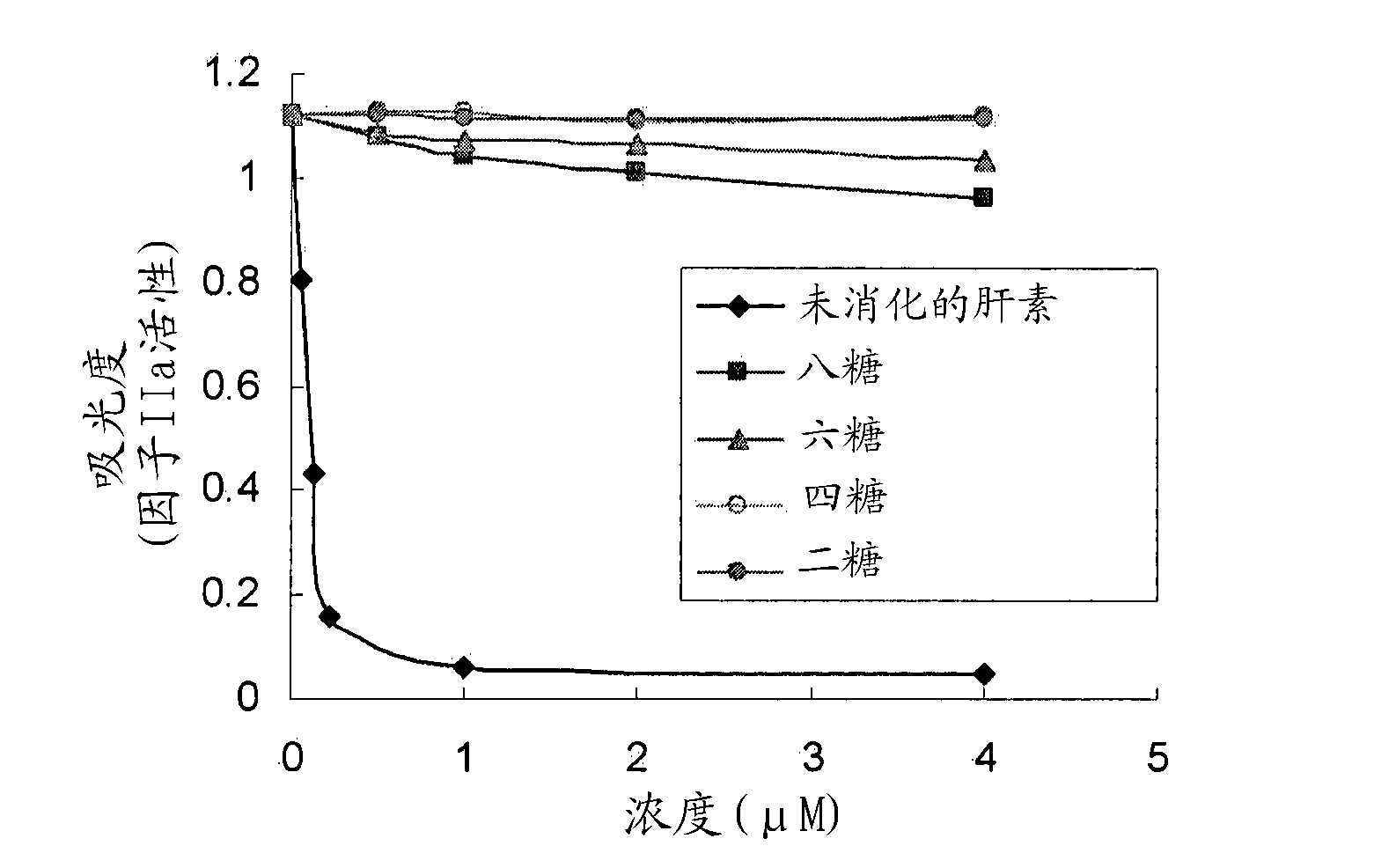
[0095] The commercially available porcine mucosal heparin was digested into smaller heparin fragments with heparinase-III. The enzymatic digestion products are then classified according to the hydrodynamic size of gel filtration (Figure 1). Four peaks were obtained and determined to contain disaccharides, tetrasaccharides, hexasaccharides and octasaccharides, as shown in Table 1 below. The total heparin concentration (measured by absorbance at 232 nm) divided by the concentration of glucuronic acid (GlcA) (measured by the carbazole method) is used to determine the length of heparin saccharides.
[0096] Table 1: Characterization of heparin oligosaccharides after heparinase-III digestion
[0097] Heparin oligosaccharide concentration (mM) GlcA concentration (mM) GlcA number per chain (GlcA concentration / heparin oligosaccharide concentration) Heparin oligosaccharide length Peak I13.265.34.9 Octa & higher sugar (Octa &...
Embodiment 2
[0099] Example 2: Preparation of chitosan beads loaded with heparin fragments
[0100] Through Wang et al., J Control. Release 106:62-75 (2005) described the SPG membrane emulsification technology to prepare five micrometer (5 μm) chitosan beads. Briefly, a chitosan solution was prepared by dissolving 1.6% chitosan in 1% (w / v) acetic acid and 5% (w / v) sodium chloride. Then pour 2ml of chitosan solution into a Teflon tank as the dispersed phase (aqueous phase), and allow it to pass through the SPG membrane (pore size 5μm) under pressure (16Kpa) at 600rpm to 4% ( w / v) Tween® 80 is used as an emulsifier in a continuous oil phase for 2 hours to form a W / O emulsion. The chitosan beads were cured by adding a sodium hydroxide mixture containing 100 μl 10M NaOH, 100 μl Tween® 80 and 4 ml oil. The chitosan beads were then collected and washed with 1% Tween 80 twice, followed by distilled water three times. The chitosan beads were then air dried and stored at room temperature. 9.8 ng ...
Embodiment 3
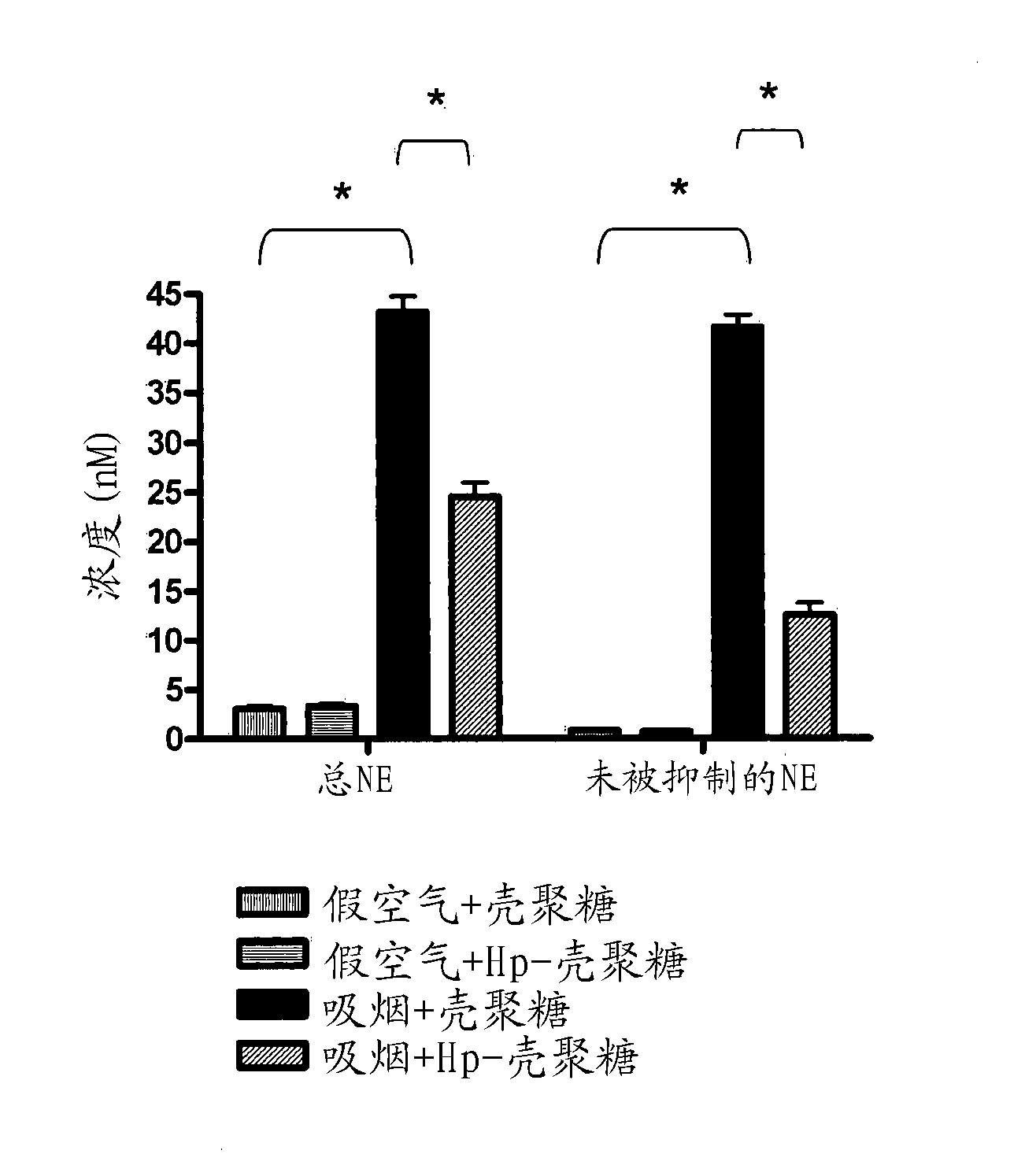
[0101] Example 3: Treatment of chitosan loaded with heparin derivatives reduces the immune response of rats exposed to cigarette smoke
[0102] Materials and Method
[0103] Smoking rat model
[0104] The Sprague-Dawley rats were divided into two groups, cigarette smoke group and fake air group. In the cigarette smoke group (8 rats), they were exposed to the smoke of 11 cigarettes (1hr per day for 4 weeks) in a ventilated smoking room. The dummy air group (8) was placed in the device for the same period of time, but was exposed to indoor air instead. After 4 weeks of exposure to cigarette smoke or fake air, 4 rats in each group were treated with 2 mg of heparin derivative-loaded chitosan (HP-chitosan). Each rat is about 400g. Rats were anesthetized with pentobarbital, and 2 mg HP-chitosan / rat was administered intratracheally using a DP-4 dry powder insufflator® device (PennCentury Inc., Philadelphia, Pennsylvania, USA). The remaining four rats in each group were given pure chi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com