Preparation method of indium electrolyte solution
A preparation method and electrolyte technology, applied to the improvement of process efficiency, photographic technology, instruments, etc., can solve the problems of long time, unqualified products, high indium impurity content, etc., and achieve long service life, small indium loss, and residual impurities low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
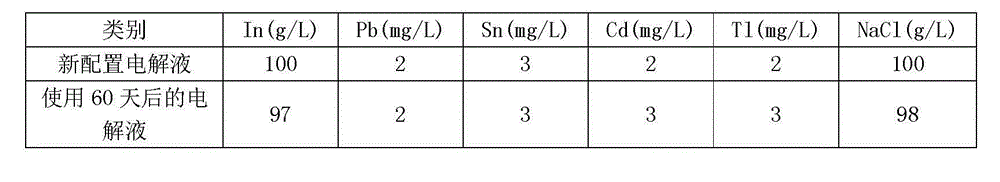
[0026] Add 5kg of refined indium with a purity of 99.99% plus 6.4kg (3.5L) of concentrated sulfuric acid with a purity of analytical grade, then add 46.5kg of pure water, heat in a container (titanium tank) at 60°C and stir with a glass rod to dissolve the indium Finally, the initial electrolyte solution with a concentration of 100g / L was obtained; then, the initial electrolyte solution obtained in step A was adjusted to pH 1.5 with 99.99% solid sodium hydroxide (NaOH) solution, and then 99.99% solid sodium chloride was added in sequence 5kg, 25g of 99% solid gelatin, and 20g of 99.99% solid thiourea were dissolved to obtain the final indium electrolyte, and the pure water was pure water with a conductivity of 18.25 MΩ / cm.
example 2
[0028] Add 10kg of refined indium with a purity of 99.99% to 12.8kg (7L) of concentrated sulfuric acid with a purity of analytical grade, then add 93kg of pure water, heat and dissolve in the container at 80°C, and obtain an initial electrolyte solution with a concentration of 100g / L Then, adjust the pH value to 2 with the initial electrolyte solution that step A obtains, then add solid sodium chloride 10kg, gelatin 50g, solid thiourea 40g successively, after dissolving, obtain final electrolyte , and the rest are the same as Example 1.
example 3
[0030] Add 20kg of refined indium with a purity of 99.99% to 25.6kg (14L) of concentrated sulfuric acid with a purity of analytical grade, then add 186kg of pure water, heat and dissolve in the container at 95°C, and obtain an initial electrolyte solution with a concentration of 100g / L Then, adjust the pH value to 3.5 with the initial electrolytic solution that step A obtains, then add solid sodium chloride 20kg, gelatin 100g, solid thiourea 40g successively, after dissolving, obtain final electrolytic solution , and the rest are the same as Example 1.
[0031] Sodium oxide, gelatin, and sulfuric acid are all produced by Shanghai Sinopharm Group and are of superior grade; refined indium is produced by the company itself and extracted from crude indium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com