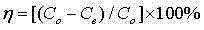Method for removing fluorine in bastnaesite sulfuric acid leaching liquid by using zirconium-containing adsorbent
A technology of bastnaesite and adsorbent, applied in the field of rare earth hydrometallurgy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
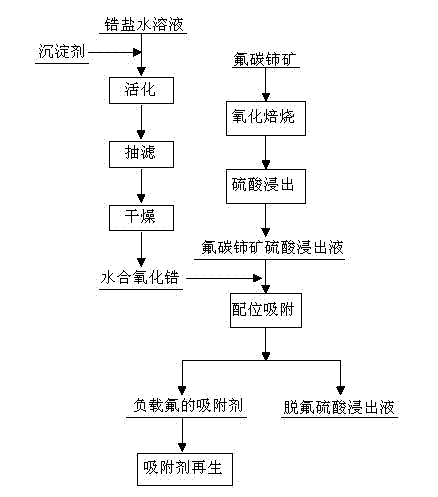
[0030] (1) ZrOCl 2 Dissolve in water to prepare 0.1mol L -1 solution, add 0.1mol·L while stirring -1 NaOH solution was used as a precipitating agent, and the pH was adjusted to 9. After the white precipitate was formed, it was stirred and activated at 65°C for 4h, and then filtered with suction. The obtained solid product was washed with distilled water until it was neutral, and dried at 100°C for 4h to obtain a zirconium-containing adsorbent. hydrated zirconia;
[0031] (2) The bastnaesite was oxidized and roasted at 400°C for 4 hours, and the concentration of 0.5mol L was added to the oxidized and roasted bastnaesite -1 sulfuric acid, leached at 50°C for 4h, the liquid-solid weight ratio of sulfuric acid to bastnaesite was 5:1, to obtain bastnaesite sulfuric acid leaching solution;
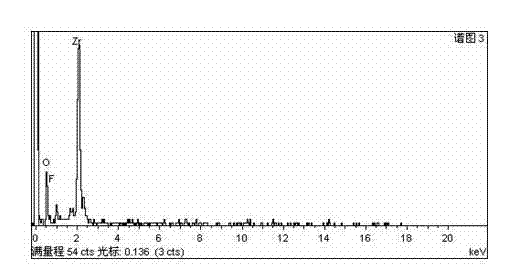
[0032](3) Dilute bastnaesite sulfuric acid leaching solution with water 10 times, where F - The concentration is 4.3×10 -3 mol L -1 , Ce 4+ The concentration is 2.6×10 -3 mol L -1 , RE ...
Embodiment 2
[0036] (1) ZrO(NO 3 ) 2 Dissolve in water to make 0.05mol·L -1 solution, add 0.05mol·L while stirring -1 NaOH solution was used as a precipitating agent, and the pH was adjusted to 10. After the white precipitate was formed, it was stirred and activated at 75°C for 2h, and then filtered with suction. hydrated zirconia;
[0037] (2) The bastnaesite was oxidized and roasted at 500°C for 3 hours, and the concentration of 2.0mol L was added to the oxidized and roasted bastnaesite -1 sulfuric acid, leached at 65°C for 2h, the liquid-solid weight ratio of sulfuric acid to bastnaesite was 10:1, to obtain bastnaesite sulfuric acid leaching solution;
[0038] (3) Dilute bastnaesite sulfuric acid leaching solution with water 30 times, where F - The concentration is 7.23×10 -3 mol L -1 , Ce 4+ The concentration is 4.96×10 -3 mol L -1 , RE 3+ The concentration is 7.4×10 -3 mol L -1 , adjust the acidity to 1mol L -1 , adding 1.0 g / 50 ml of the prepared zirconium-containing ad...
Embodiment 3
[0042] (1) ZrCl 4 Dissolve in water to prepare 0.5mol L -1 solution, add 0.5mol L while stirring -1 The ammonia solution was used as a precipitating agent, and the pH was adjusted to 10. After the white precipitate was formed, it was stirred and activated at 100 ° C for 1 h, and then filtered with suction. hydrated zirconia;
[0043] (2) The bastnaesite was oxidized and roasted at 800°C for 1 hour, and the concentration of 6.0mol L was added to the oxidized and roasted bastnaesite -1 Sulfuric acid was leached at 100°C for 0.5h, the liquid-solid weight ratio of sulfuric acid to bastnaesite was 1:1, and bastnaesite sulfuric acid leach solution was obtained;
[0044] (3) Dilute bastnaesite sulfuric acid leaching solution with water 100 times, where F - The concentration is 5.2×10 -3 mol L -1 , Ce 4+ The concentration is 2.89×10 -3 mol L -1 , RE 3+ The concentration is 5.02×10 -3 mol L -1 , adjust the acidity to 1mol L -1 , adding 0.4g / 50ml of the prepared zirconium-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com