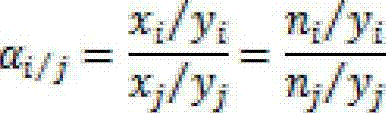Metal-organic framework material for methane adsorption separation and preparation method thereof
A metal organic framework, adsorption separation technology, applied in separation methods, dispersed particle separation, chemical instruments and methods, etc., can solve problems such as uncontrollable, complex surface functional groups, and separation efficiency cannot be further enhanced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 131.4g 2-methylimidazole (2-mIM) and dissolve in 830ml deionized water to form solution A; 57.5g ZnSO 4 ·7H 2O was dissolved in 250 ml of deionized water to form solution B. Add solution B to solution A while stirring constantly to form a homogeneous mixture. Add 48g of triethylamine into 50ml of methanol, and add it into the mixed solution under stirring to form mixed solution C. Transfer the mixture C into a PTFE-lined 2L reactor, continue to react at 100°C for 2h, then gradually cool to room temperature, filter, wash with deionized water for 3 times, and dry the precipitate at 105°C Slightly yellowish crystals were obtained in 4 hours. After drying, 45 g of the obtained product was added, and 1% graphite and 3% polyvinylpyrrolidone were added to mix evenly. After molding, the adsorbent A was formed after vacuum activation at 130° C. for 4 hours.
[0036] By measuring the breakthrough curve of components i and j in the mixed gas, the adsorption amount of eac...
Embodiment 2
[0042] Take by weighing 70.2g copper acetate (Cu(CH 3 OO) 2 .H 2 O), 41.2g of trimesic acid was dissolved in 500ml of DMF, and stirred to form a homogeneous mixture. Under normal pressure and stirring conditions, the reaction was continued at 110°C for 8h, then gradually cooled to room temperature, filtered, washed twice with 200ml of methanol, and the precipitate was dried at 80°C for 6 hours to obtain dark blue crystals. After drying, 60 g of the obtained product was pulverized, and 2% graphite and 4% polyvinylpyrrolidone were added and mixed uniformly. After molding, the adsorbent B was formed after vacuum activation at 180° C. for 8 hours.
[0043] Comparative test, according to the method described in BASF patent CN101384537A: 12.2g of 1,3,5-BTC and 13.9g of anhydrous copper sulfate were suspended in 275g of ethylene glycol, and kept under stirring at 100°C for 8 hours . The blue precipitate was filtered off and washed with 5 x 120 ml methanol. Dry at 75°C and vacuum...
Embodiment 3
[0049] Weigh 30g of copper acetate, 21g of trimesic acid and dissolve in 500ml of ethanol water (volume ratio 1:1), stir to form a uniform mixture. Transfer the mixed solution into a PTFE-lined 1L reaction kettle, continue the reaction at 110°C for 4h, then gradually cool to room temperature, then gradually cool to room temperature, filter, wash twice with 75ml ethanol, and remove the precipitate at 80°C C dried for 6 hours to obtain blue crystals. After drying, 29 g of the obtained product was pulverized, and 2% graphite and 4% polyvinylpyrrolidone were added to mix evenly. After molding, the adsorbent D was formed after vacuum activation at 130° C. for 4 hours.
[0050] N of the obtained adsorbent D 2 The specific surface area (Langmuir method) is 1982m 2 / g, the average pore size is 0.7nm;
[0051] The obtained adsorbent D is at 298K, between 0-1Mpa,
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com