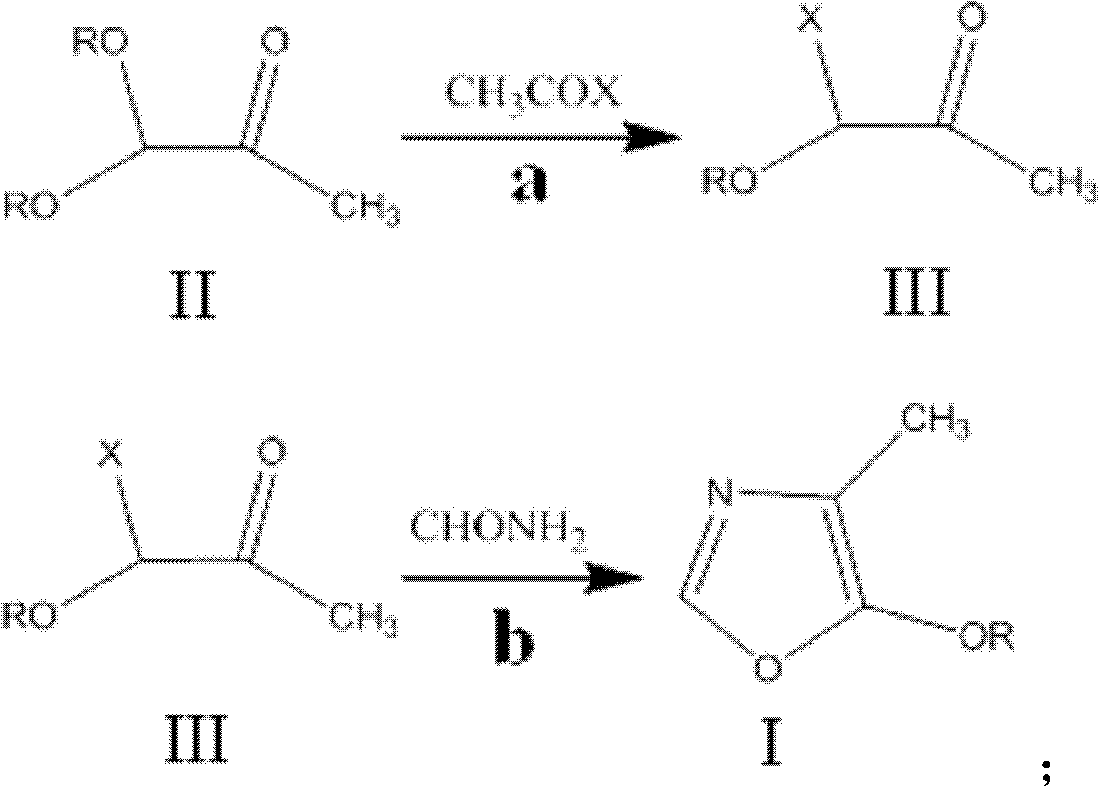
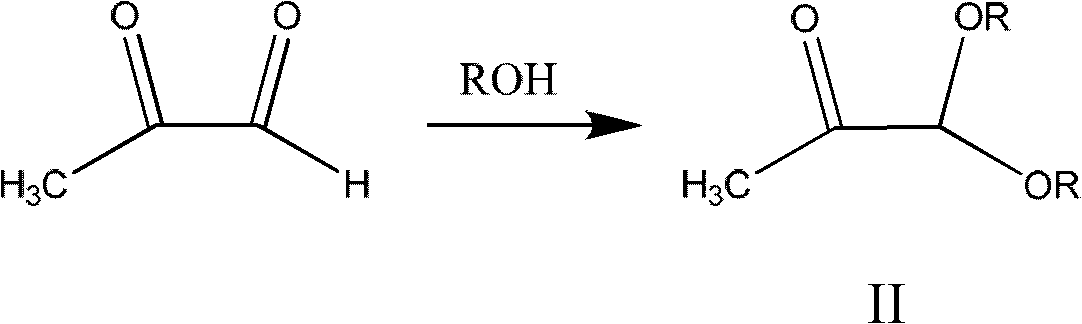
Method for synthesizing 4-methyl-5-alkoxyl oxazole
A technology of alkoxy oxazole and alkoxy, which is applied in the field of organic chemical synthesis, can solve the problems that are not suitable for the development requirements of energy-saving and environmental protection industries, cannot meet the requirements of industrialized large-scale production, and have many by-products and three wastes, etc., and achieve low toxicity and low pollution , the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Synthesis of intermediate 1-bromo-1-n-butoxyacetone
[0026] Under argon protection, at first 24.3g of acetyl bromide and 40g of 1,1-di-n-butoxyacetone prepared according to CA1153014A (the molar ratio of the two is 1:1) were heated to 60°C respectively, and then the acetyl bromide Add dropwise into 1,1-di-n-butoxyacetone, after dropping, continue to keep warm and stir for 1 hour, cool to 50°C, start vacuum distillation (11mmHg), gradually raise the temperature, collect fractions at 78-80°C, and obtain 37.7g The molar yield of intermediate 1-bromo-1-n-butoxyacetone relative to 1,1-di-n-butoxyacetone was 91.3%.
[0027] Two, synthetic target product 4-methyl-5-n-butoxy oxazole
[0028] Under the protection of argon, firstly, the obtained 37.7g intermediate 1-bromo-1-n-butoxyacetone was heated to an internal temperature of 130° C., and then 8.5 g of formamide (1-bromo-1-n-butoxyacetone was added dropwise. The molar ratio of methyl acetone and formamide is 1: 1.05); A...
Embodiment 2
[0031] Under the protection of argon, first 48.6g of acetyl bromide and 40g of 1,1-di-n-butoxyacetone (the molar ratio of the two is 2:1) were heated to 76°C respectively, and then the acetyl bromide was added dropwise to In 1,1-di-n-butoxyacetone, after dropping, continue to heat and stir for 1 hour, cool to 50°C, start vacuum (11mmHg) distillation, gradually raise the temperature, collect fractions at 78-80°C, and obtain 38.1g of intermediate 1 -Bromo-1-n-butoxyacetone, the molar yield is 92.2%.
[0032] Under the protection of argon, firstly, the obtained intermediate 1-bromo-1-n-butoxyacetone was heated to an internal temperature of 120° C., and then 8.2 g of formamide (1-bromo-1-n-butoxy The molar ratio of acetone to formamide is 1: 1); After dropping, continue to insulate and stir for 2 hours; Cool to 50°C, start vacuum (11mmHg) distillation, gradually heat up, collect 74~76°C fractions, and obtain the target product 4- Methyl-5-n-butoxyoxazole 26.0 g, GC purity 99.2%, ...
Embodiment 3
[0034] Under argon protection, first 15.5 g of acetyl chloride and 40 g of 1,1-di-n-butoxyacetone (the molar ratio of the two is 1:1) were heated to 51 ° C, and then the acetyl chloride was added dropwise to In 1,1-di-n-butoxyacetone, after dropping, continue to heat and stir for 1 hour, cool to 30°C, start vacuum (11mmHg) distillation, gradually raise the temperature, collect fractions at 52-54°C, and obtain 29.3g of intermediate 1 -Chloro-1-n-butoxyacetone, the molar yield is 90.0%.
[0035] Under the protection of argon, firstly, the obtained intermediate 1-chloro-1-n-butoxyacetone was heated to an internal temperature of 110° C., and then 8 g of formamide (1-chloro-1-n-butoxyacetone The molar ratio to formamide is 1:1); after dropping, continue to keep warm and stir for 2 hours; cool to 50°C, start vacuum (11mmHg) distillation, gradually heat up, collect fractions at 74-76°C, and obtain the target product 4-formamide 25.8 g of 1-5-n-butoxyoxazole, the GC purity was 98.8%,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com