Method for preparing good-stability super-hydrophobic fabrics
A super-hydrophobic and stable technology, applied in physical treatment, textiles and papermaking, fiber treatment, etc., can solve the problems of limiting the practical application of super-hydrophobic fabrics, poor stability of super-hydrophobic surfaces, and insufficient bonding of compounds and fabric fibers. , to achieve the effect of being convenient for mass production, maintaining mechanical properties and gloss, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Measure 96 μL of tetraethoxysilane and 392 μL of methyltriethoxysilane into a round-bottomed flask filled with 20 mL of saturated ethanol-ammonia solution, and stir magnetically for 10 minutes to obtain a uniform alcohol-ammonia solution of organosilane; add 1.5 mL of water, heated up to 50°C within 0.5h, and kept at this temperature for 48h; cooled to room temperature, and obtained the crude product by suction filtration; washed with 10mL of methanol and filtered three times, then vacuum dried at 50°C for 3h to obtain organosilane polymerization things. Weigh 60mg organosilane polymer, dissolve in 10mL toluene, stir magnetically for 5min, disperse ultrasonically for 5min, and then put 2×2cm 2 The polyester fabric was immersed in it, and after standing for 5 minutes, it was ultrasonically treated for 20 minutes. The fabrics were taken out to dry and evaluated for performance.
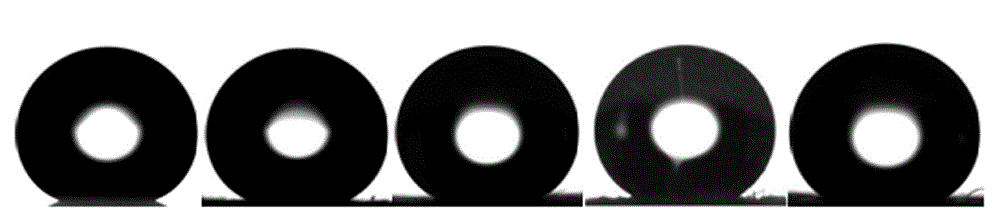
[0037] The evaluation results of the sample superhydrophobicity and stability are shown in ...
Embodiment 2
[0039]Measure 538 μL of tetraethoxysilane, 122 μL of aminopropyltrimethoxysilane and 264 μL of octyltriethoxysilane into a round bottom flask filled with 40 mL of saturated ethanol-ammonia solution, and stir magnetically for 10 minutes to obtain a uniform organic Alcohol-ammonia solution of silane; add 6.5mL of water, raise the temperature to 50°C within 0.5h, and keep the temperature for 24h; cool to room temperature, and obtain the crude product by suction filtration; °C for 3 hours in vacuum to obtain an organosilane polymer. Weigh 40 mg of the polymer, dissolve it in 5 mL of toluene, stir it magnetically for 5 min, and disperse it ultrasonically for 5 min, then place 2×2 cm 2 The cotton fabric was immersed in it, and after standing for 5 minutes, it was ultrasonically treated for 30 minutes. The fabrics were taken out to dry and evaluated for performance.
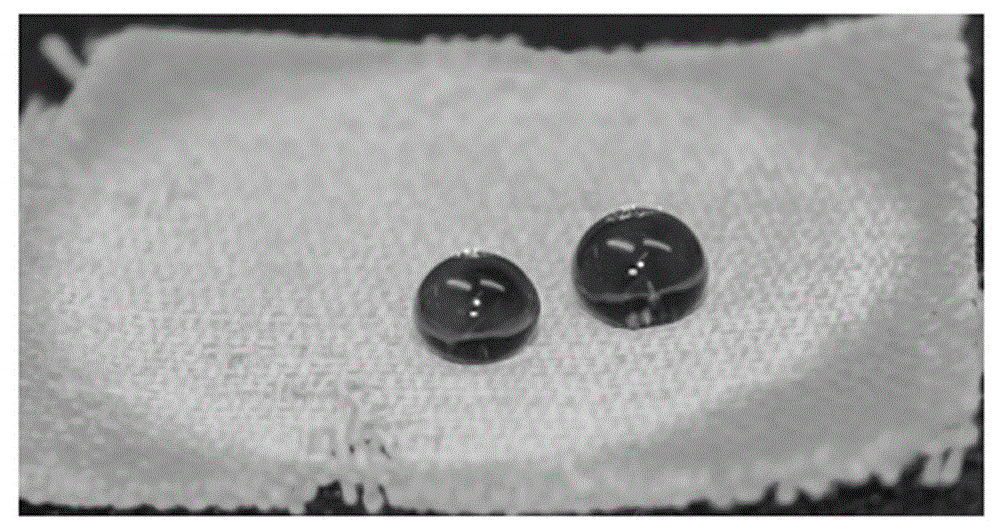
[0040] The evaluation results are shown in Table 1. For photos of samples see figure 2 , the photo after 200 fri...
Embodiment 3
[0042] Measure 282 μL of tetraethoxysilane, 118 μL of 3-(2,3-glycidoxy)propyltrimethoxysilane and 418 μL of hexadecyltriethoxysilane, and add them to 20 mL of saturated ethanol-ammonia In a round-bottomed flask of the solution, stir magnetically for 10 minutes to obtain a homogeneous alcohol-ammonia solution of organosilane; add 2.8mL of water, raise the temperature to 40°C within 0.5h, and keep the temperature for 48h; cool to room temperature, and obtain crude The product was washed with 10 mL of methanol-suction filtered three times, then dried in vacuum at 50° C. for 3 h to obtain an organosilane polymer. Weigh 40 mg of the polymer, dissolve it in 5 mL of toluene, stir it magnetically for 5 min, disperse it ultrasonically for 5 min, and then spray the solution onto 2×2 cm 2 on silk fabric. After ultrasonic treatment for 10 min, the fabric was treated at 80 °C for 1 h, and its performance was evaluated.
[0043] The evaluation results are shown in Table 1. For photos of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com