Agent for suppressing the formation of abnormal skin cells caused by exposure to light
一种皮肤细胞、异常的技术,应用在皮肤疾病、非有效成分的医用配制品、含有效成分的医用配制品等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
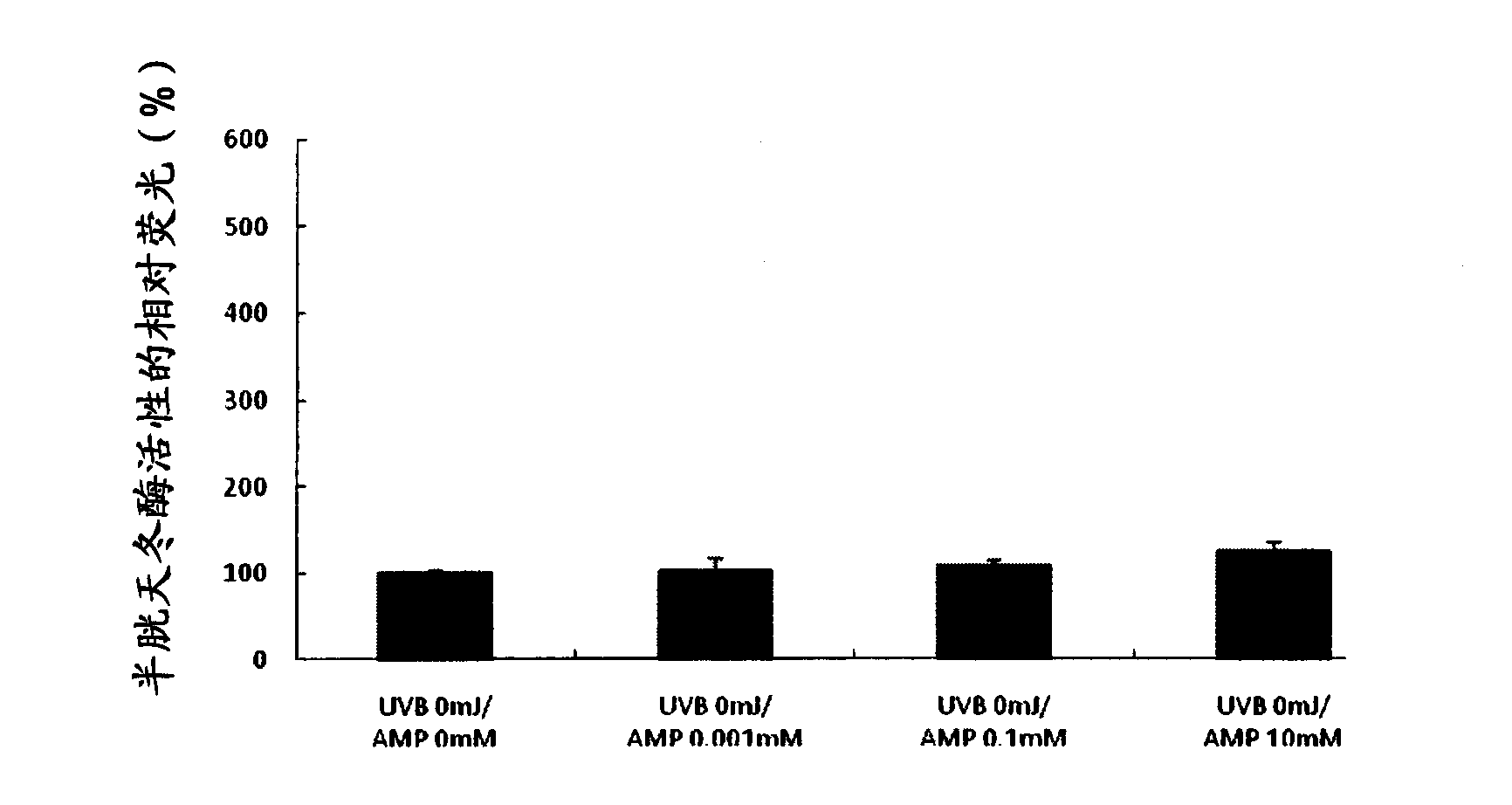
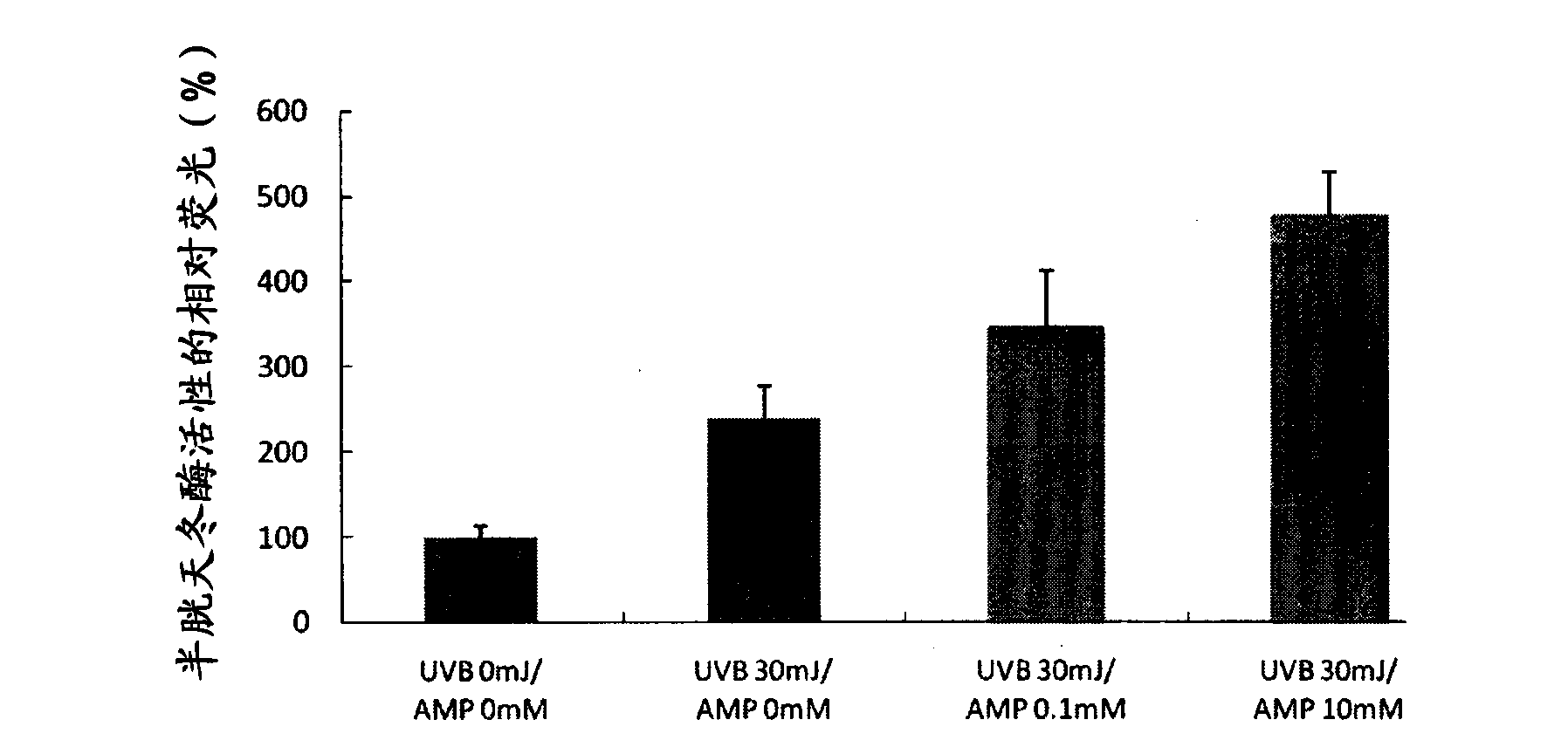
[0074] Example 1: Effect of Inducing Apoptosis in Mutant Cells Caused by Ultraviolet Light Irradiation
[0075] In this example, changes in caspase activity were detected when human epithelial cells treated with 5'-adenosine monophosphate disodium (AMP2Na) were irradiated with ultraviolet rays.
[0076]
[0077]Using EpiLife-KG2 medium (manufactured by Kurabo Industries Ltd.), human epithelial keratinocytes (purchased from Kurabo Industries Ltd. ) and seeded at a density of 30,000 cells / well into collagen-coated 96-well microplates. After culturing in EpiLIfe-KG2 medium for 8 hours, the medium was replaced with EpiLife medium (manufactured by Kurabo Industries Ltd.), followed by further culturing for 16 hours. Subsequently, the medium was replaced with AMP2Na-containing medium adjusted to various concentrations. After being treated with the culture medium for 2 hours, the cells were washed with PBS (phosphate buffered saline) at 30 mJ / cm using an ultraviolet light irradi...
Embodiment 2
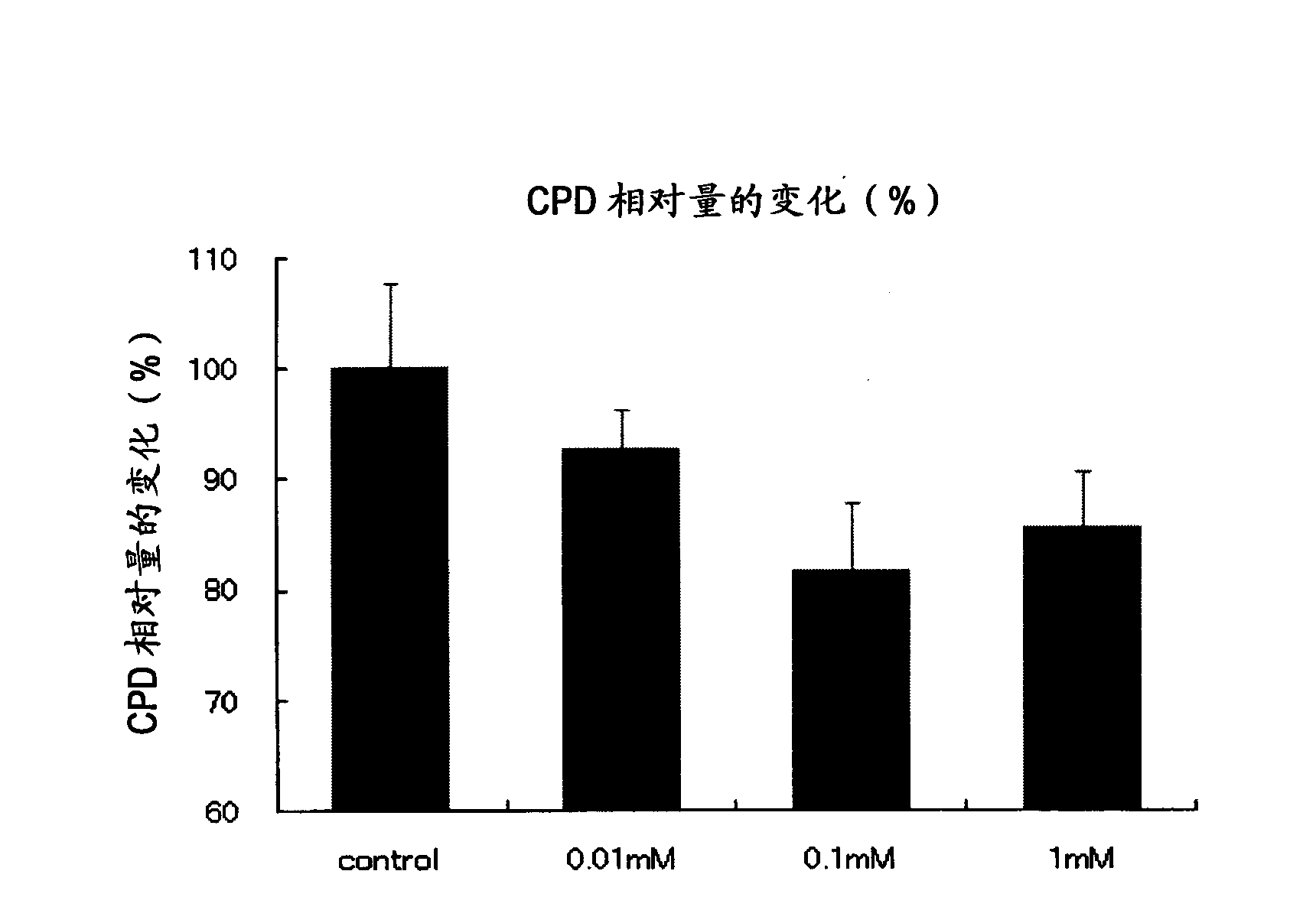
[0082] Example 2: Evaluation of the Effect on Reducing DNA Mutations Induced by UV Light Irradiation
[0083] In this example, normal mouse epithelial cells were irradiated with ultraviolet rays and then cultured in a medium containing AMP2Na. The effect of AMP2Na on reducing DNA mutations was examined using the amount of cyclobutane pyrimidine dimer (CPD) as an index.
[0084]
[0085] JB6 cells (purchased from ATCC) derived from normal mouse epithelial cells were cultured in FBS-containing MEM medium until confluent. Subsequently, the 4×10 5 The cultured cells were seeded into a 3.5 cm dish with MEM (minimum essential medium) containing FBS (fetal bovine serum). The day after inoculation, the medium was replaced by serum-free MEM medium for serum starvation. Subsequently, the medium was replaced by PBS, followed by 15 mJ / cm 2 of UV-B irradiation. After UV irradiation, the medium was replaced with serum-free MEM medium containing 0.01 mM, 0.1 mM or 1 mM AMP2Na or med...
Embodiment 3
[0091] Example 3: Evaluation of Preventive Effects on Skin Cancer in Ultraviolet-Irradiated Mice
[0092] In this example, hairless mice were irradiated with ultraviolet rays, and the preventive effect of AMP2Na against photocarcinogenesis in mice was tested.
[0093]
[0094] 5-week-old female Hos:HR-1 mice were purchased from Japan SLC, Inc., and their backs were irradiated with ultraviolet (UV--B) from 7 weeks of age. Ultraviolet light irradiation (each irradiation dose: 60mJ / cm 2 ) once a day, five days a week for 12 weeks.
[0095] Then, a 20% ethanol aqueous solution (test solution) containing 3% AMP2Na dissolved therein and a 20% ethanol aqueous solution (substrate) not containing AMP2Na were prepared.
[0096] After feeding the ultraviolet-irradiated mice under normal conditions for 1 week, the mice were divided into 3 groups (6 mice per group) under the conditions shown in Table 1 and fed for 12 weeks.
[0097] Table 1
[0098]
[0099]
[0100] The condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com