Pharmaceutical composition containing adenosine cyclophosphate compound and preparation method of pharmaceutical composition
A cyclic adenosine monophosphate compound and the technology of cyclic adenosine monophosphate, which are applied in the field of pharmaceutical compositions containing cyclic adenosine monophosphate compounds and their preparation, can solve the problems of poor water solubility, poor preparation stability, and poor resolubility of cyclic adenosine monophosphate, etc. Achieve the effect of good stability and resolubility, improved water solubility and simple formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
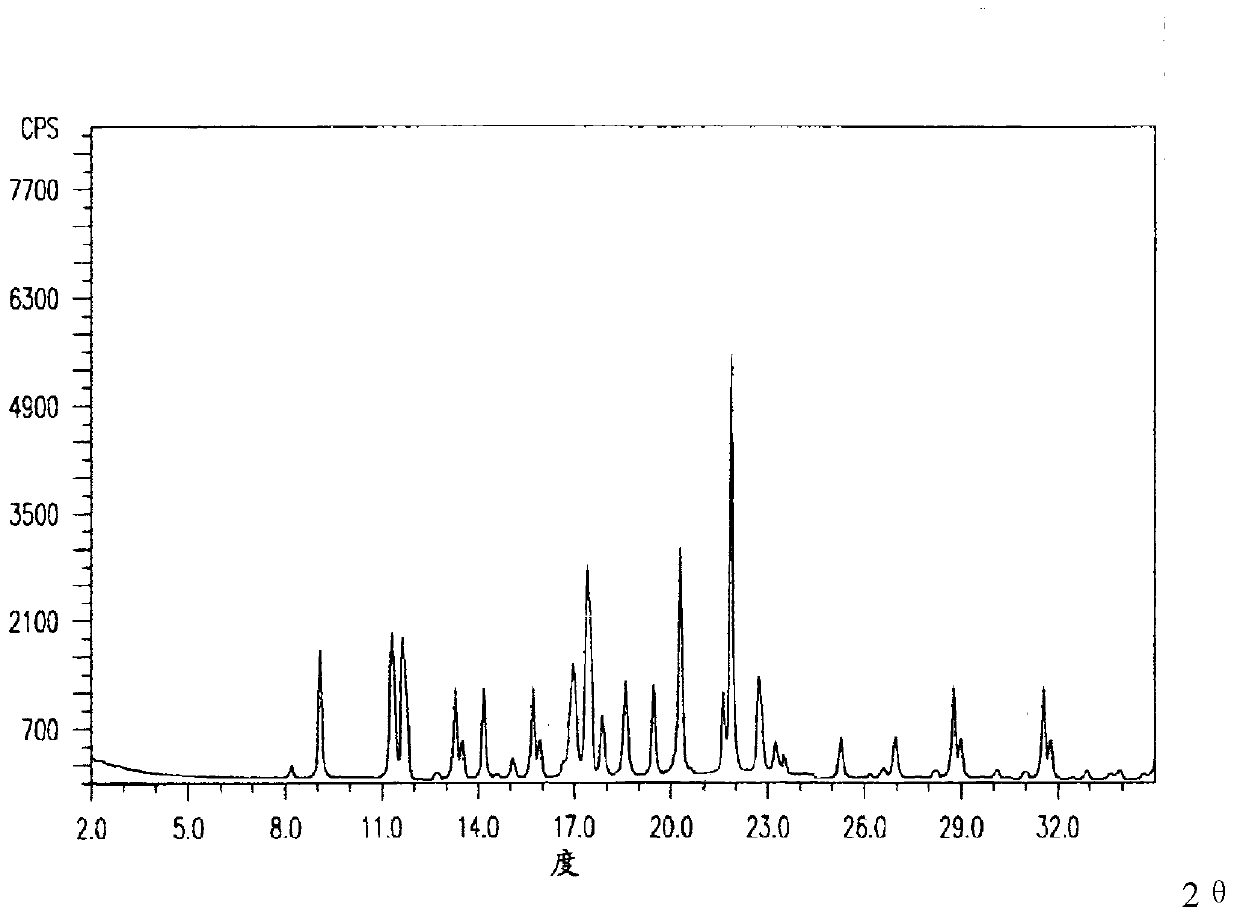
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation method of crystal compound
[0030] 1. Prepare a solution according to the ratio of adding 40g cyclic adenosine monophosphate solid per liter of water, heat to 45°C, and stir until completely dissolved;
[0031] 2. Under a sound field with a frequency of 20KHz and an output power of 30W, add dropwise a mixed solvent of methanol, isopropanol and ether at 1°C while stirring, and the stirring speed is 120 rpm; the methanol, isopropanol and ether The volume ratio of the solvent is 2:2:1, and the volume of the mixed solvent of methanol, isopropanol and ether is twice that of the aqueous solution of cyclic adenosine monophosphate; the speed of adding the mixed solvent is 240ml / min;
[0032] 3. After adding the mixed solvent, stop the sound field and stirring, continue to cool down to 1°C, and let the crystal grow for 3 hours; after obtaining the crystal, filter it, wash it with absolute ethanol, and dry it in vacuum for 4 hours to obtain a crysta...
Embodiment 2
[0035] Embodiment 2: the preparation method of crystal compound
[0036] 1. Prepare the solution according to the ratio of adding 40g cyclic adenosine monophosphate solid per liter of water, heat to 60°C, and stir until completely dissolved;
[0037] 2. Under a sound field with a frequency of 25KHz and an output power of 60W, a mixed solvent of methanol, isopropanol and ether at 5°C was added dropwise while stirring, and the stirring speed was 180 rpm; the volume of the methanol, isopropanol and ether The ratio is 2:1:1; the volume of the mixed solvent of methanol, isopropanol and ether is 4 times that of the aqueous solution of cyclic adenosine monophosphate; the speed of adding the mixed solvent is 200ml / min;
[0038] 3. After the mixed solvent was added, the sound field and stirring were stopped, and the temperature was continued to drop to 5°C, and the crystal was left standing for 8 hours; after the crystal was obtained, it was filtered, washed with absolute ethanol, a...
Embodiment 3
[0040] Embodiment 3: the preparation method of crystal compound
[0041] 1. Prepare a solution according to the ratio of adding 42g cyclic adenosine monophosphate solid per liter of water, heat to 50°C, and stir until completely dissolved;
[0042] 2. Under a sound field with a frequency of 2KHz and an output power of 40W, add dropwise a mixed solvent of methanol, isopropanol and ether at 2°C while stirring, and the stirring speed is 160 rpm; the methanol, isopropanol and ether The volume ratio of the solvent is 4:2:1; the volume of the mixed solvent of methanol, isopropanol and ether is 3 times that of the aqueous solution of cyclic adenosine monophosphate; the speed of adding the mixed solvent is 100ml / min;
[0043] 3. After adding the mixed solvent, stop the sound field and stirring, continue to cool down to 1-5°C, and let the crystal grow for 3-8 hours; after obtaining the crystal, filter it, wash it with absolute ethanol, and dry it in vacuum for 4-8 hours to obtain Cyclo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Master granularity | aaaaa | aaaaa |
| Master granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com