Method for producing N,N'-dimethyl cyclohexanediamine
A technology of dimethylcyclohexanediamine and its production method, which is applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., to achieve the effects of low raw material cost, non-toxic production process and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
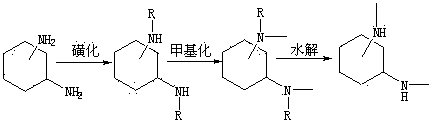
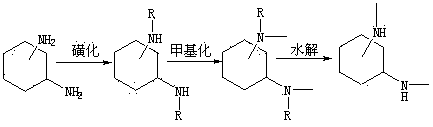
[0019] Step 1: Sulfonation of Cyclohexanediamine
[0020] Dissolve 20mmol of 1,2-cyclohexanediamine in 10ml of pyridine, add the above solution dropwise into 10ml of pyridine solution mixed with 40mmol of p-toluenesulfonyl chloride, control the temperature below 60°C during the dropwise addition, when the color of the solution When it changes from colorless to dark red, after dropping, put it at room temperature to react for 7 hours, stop the reaction, add 15% hydrochloric acid to adjust the pH to 6-7, let it stand for 24 hours, filter, and wash the solid with 10ml of water for 3 times, 6.5 g of a white powdery solid were obtained.
[0021] Step 2: Methylation
[0022] Dissolve 7.18mmol of the product of step 1 in 60ml of acetonitrile, add 28.7mmol of potassium carbonate powder, stir at room temperature for 1h, add 28.7mmol of methyl iodide in an ice bath, react in an ice bath for 30min, remove the ice bath and then return to room temperature React for 12 hours, stop the rea...
Embodiment 2
[0026] Step 1: Sulfonation of Cyclohexanediamine
[0027] Dissolve 20mmol of 1,4-cyclohexanediamine in 10ml of pyridine, add the above solution dropwise into 10ml of pyridine solution mixed with 40mmol of p-toluenesulfonyl chloride, control the temperature below 60°C during the dropwise addition, when the color of the solution When it changes from colorless to dark red, after dropping, put it at room temperature to react for 7 hours, stop the reaction, add 15% hydrochloric acid to adjust the pH to 6-7, let it stand for 24 hours, filter, and wash the solid with 10ml of water for 3 times, 7.3 g of a white powdery solid were obtained.
[0028] Step 2: Methylation
[0029] Dissolve 7.18mmol of the product of step 1 in 60ml of acetonitrile, add 28.7mmol of potassium carbonate powder, stir at room temperature for 1h, add 28.7mmol of methyl iodide in an ice bath, react in an ice bath for 30min, remove the ice bath and then return to room temperature React for 12 hours, stop the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com