Lipase mutant with increased optimum temperature and application of lipase mutant with increased optimum temperature
An optimum temperature, lipase technology, applied in the field of lipase mutants, can solve problems such as limiting the scope of application and reducing catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The lipase mutant that embodiment 1 optimum temperature improves
[0031] A lipase mutant with improved optimum temperature, the 310th amino acid of the lipase lipase gene sequence published in GenBank EF405962 is mutated from Asp of the parent lipase to Val.
Embodiment 2
[0032] Example 2. Yeast expressing lipase Asp310Val point mutant.
[0033] The lipase mutant gene of the present invention can be obtained by chemical total synthesis or PCR method, and the PCR method is taken as an example below to introduce:
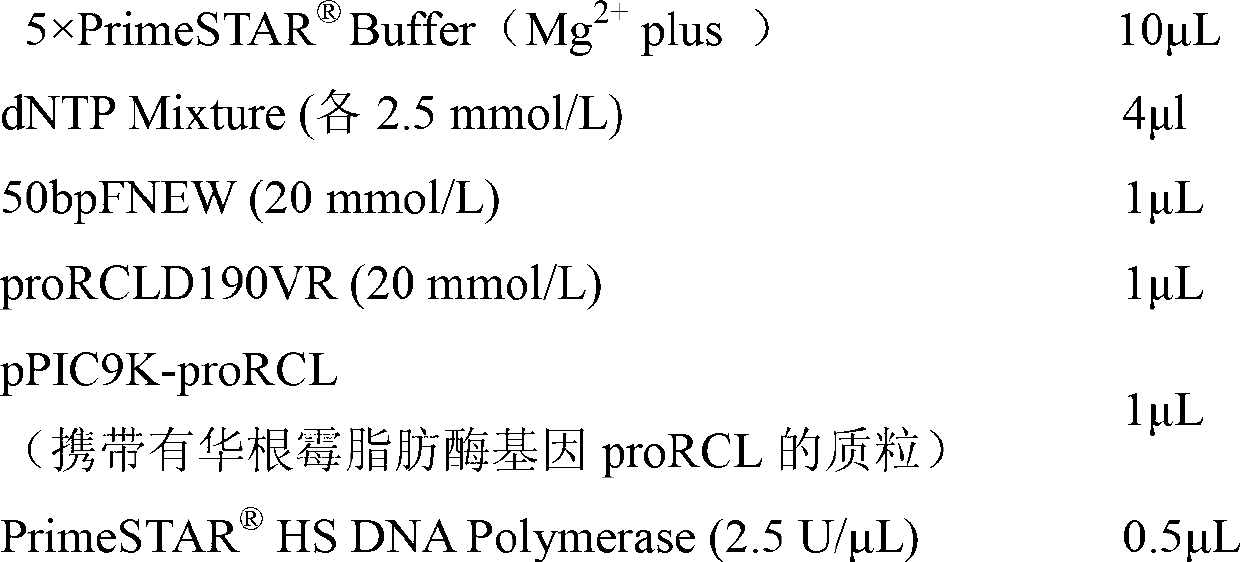
[0034] Nucleotide mutations were introduced into the lipase gene proRCL of Rhizopus sinensis in vitro by overlap extension PCR. The reaction conditions are as follows:
[0035] Reaction condition 1:
[0036]
[0037]
[0038] Among them, the sequences of the upstream primer 50bpFNEW and the downstream primer proRCLD190VR are:
[0039] 50bpFNEW:5'-AAAGAAGAAGGGGTATCTCTC-3';
[0040] proRCLD190VR:5'-TTCCGGTGCTAACAACGTAGTAAG-3'.
[0041] PCR amplification conditions: 98°C 10s; 98°C 10s, 56°C 15s, 72°C 1min, 30 cycles; 72°C 10min. The amplified product was purified by a DNA purification kit to obtain Fragment A.
[0042] Reaction condition 2:
[0043]
[0044] Among them, the sequences of the upstream primer 50bpRNEW and the...
Embodiment 3
[0051] The optimal temperature determination of embodiment 3 lipase mutants
[0052] In order to determine the optimum temperature of lipase, it is necessary to separate and purify the enzyme.
[0053] Shake flask fermentation: inoculum size 10% (V / V), in 25mL BMGY medium, shaking culture at 30°C for 16-20h to OD 600 2 to 6, the cells were collected by centrifugation and diluted to OD with BMMY medium 600 1, add 0.5% methanol every 24h to induce expression, after 3-4d of culture, collect the fermentation supernatant.
[0054] Separation and purification: the fermentation supernatant of the mutant strain is concentrated through a 10KD ultrafiltration membrane, followed by SP-Sepharose FF strong cation exchange chromatography and Phenyl-Sepharose 6FF hydrophobic chromatography column chromatography to obtain the purified active component of mutant lipase. Specific operation reference Yu Xiao-Wei et al.J Mol Catal B:Enzym,2009,57:304-311.
[0055] Determination of optimum temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com