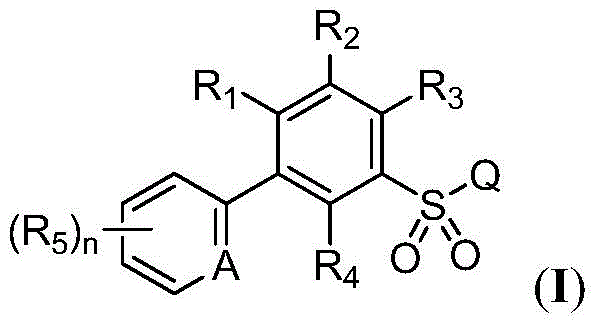
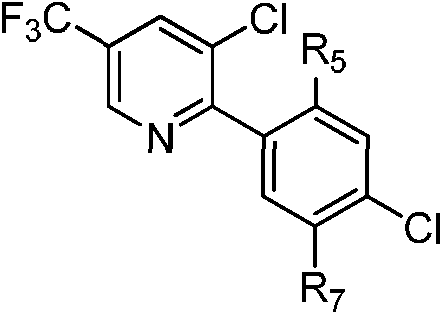
Substituted biaryl benzenesulfonamide compound and use
A kind of benzene sulfonamide and compound technology, applied in the field of substituted biaryl benzene sulfonamide compounds, can solve the problem that the herbicidal activity is not always satisfactory and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1: Ethyl 2-chloro-5-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)benzenesulfonamidoformate (Compound 44)
[0148]
[0149] The first step reaction: 3-chloro-2-(4-chlorophenyl)-5-trifluoromethylpyridine
[0150] 21.5g (0.10mol) 2,3-dichloro-5-trifluoromethylpyridine, 14.04g (0.09mol) 4-chlorophenylboronic acid, 0.5g (0.44mmol) tetrakistriphenylphosphine palladium and 41.4g ( 0.30 mol) of potassium carbonate was refluxed in a mixture of 100 ml of toluene and 50 ml of water for 6 hours, monitored by a TLC board, and the reaction of the raw material 4-chlorophenylboronic acid was completed. The reaction mixture was cooled to room temperature, filtered to obtain a filtrate, extracted with (3x80ml) ethyl acetate, combined extracts, washed with (3x60ml) water, washed with 60ml saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain a milky white solid 20.4g, yield: 70%, melting point 72-74°C.
[0151] NMR d...
Embodiment 2
[0165] Example 2: Methyl 2-chloro-5-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)-4-fluorobenzenesulfonyl formate (Compound 22)
[0166]
[0167] The first step reaction: 3-chloro-2-(4-chloro-2-fluorophenyl)-5-trifluoromethylpyridine
[0168] 21.5g (0.10mol) 2,3-dichloro-5-trifluoromethylpyridine, 15.66g (0.09mol) 4-chloro-2-fluorophenylboronic acid, 0.5g (0.44mmol) tetrakistriphenylphosphine palladium And 41.4g (0.30mol) potassium carbonate in the mixture of 100ml toluene and 50ml water reflux reaction for 6 hours, TLC plate monitoring, raw material 4-chloro-2-fluorophenylboronic acid reaction is complete. The reaction mixture was cooled to room temperature, filtered to obtain a filtrate, extracted with (3x80ml) ethyl acetate, combined extracts, washed with (3x60ml) water, washed with 60ml saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain a milky white solid 18.1g, yield: 65%, melting point 34-36°C.
[0169] N...
Embodiment 3
[0183] Example 3: 2-chloro-5-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)-N-(cyanomethyl)benzenesulfonamide (Compound 2)
[0184]
[0185] At room temperature, 0.17 g (3 mmol) of 2-aminoacetonitrile was dissolved in 15 ml of tetrahydrofuran, and 0.6 g (1.5 mmol) of 2-chloro-5-(3-chloro-5-( Trifluoromethyl)pyridin-2-yl)benzene-1-sulfonyl chloride, then triethylamine (0.4g, 4mmol) was added to the above mixture to obtain 0.25g of the title compound, yield: 40%.
[0186] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0187] δppm 3.87 (2H, d), 5.66 (1H, t), 7.70 (1H, d), 7.95 (1H, d), 8.08 (1H, s), 8.51 (1H, s), 8.86 (1H, s).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com