Osmotic pump controlled release tablet of losartan potassium and hydrochlorothiazide solid dispersion or inclusion compound
A technology for controlled release of losartan potassium hydrochlorothiazide and osmotic pump, which is applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, drug combinations, etc., can solve problems such as increasing technical complexity, and achieve high application value, prolong the effective action time, and improve the effect of dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
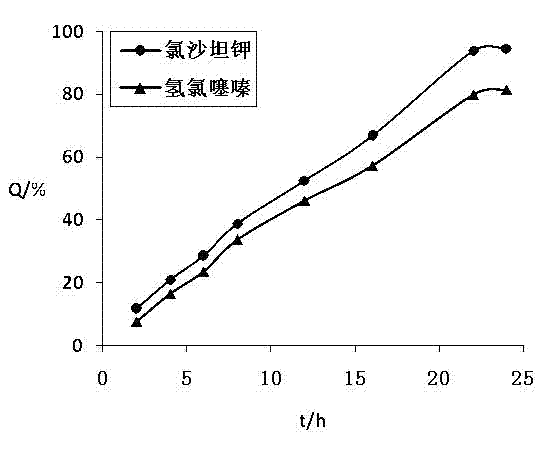
[0082] The losartan potassium hydrochlorothiazide osmotic pump controlled-release tablet is composed of a tablet core and a coating film wrapping the tablet core, and a drug release hole of 0.3-0.9 mm is punched in the center of the coating side.
[0083] The tablet core consists of the following components:
[0084] Losartan Potassium 50mg
[0085] Hydrochlorothiazide-urea solid dispersion 87.5 mg
[0086] Lactose 125 mg
[0087] Sucrose 187.5 mg
[0088] It also contains magnesium stearate accounting for 1% of the mass of the tablet core, and povidone in a dose sufficient to bind the above substances;
[0089] The mass ratio of hydrochlorothiazide to urea in the hydrochlorothiazide-urea solid dispersion is 1:6.
[0090] The used coating liquid of described coating accounts for 3~5% of tablet core weight, and each component ratio of coating liquid is:
[0091] 30 parts of cellulose acetate,
[0092] Polyethylene glycol-4000 1.2 parts,
[0093] 6 parts of diethyl ph...
Embodiment 2
[0109] The hydrochlorothiazide-urea solid dispersion in Example 1 was replaced with the hydrochlorothiazide-polyvidone solid dispersion, and the others were the same as in Example 1.
[0110] The preparation method of the hydrochlorothiazide-povidone solid dispersion is as follows: weigh hydrochlorothiazide, povidone and poloxamer 188, place them in a container, add 2~5ml / 10mg of hydrochlorothiazide dehydrated alcohol, 35~45°C Stir to dissolve completely, evaporate the solvent in a water bath at 75-85°C to obtain a dry solid, then dry it in a desiccator for 24 hours, pass through a 60-mesh sieve, and seal it for later use.
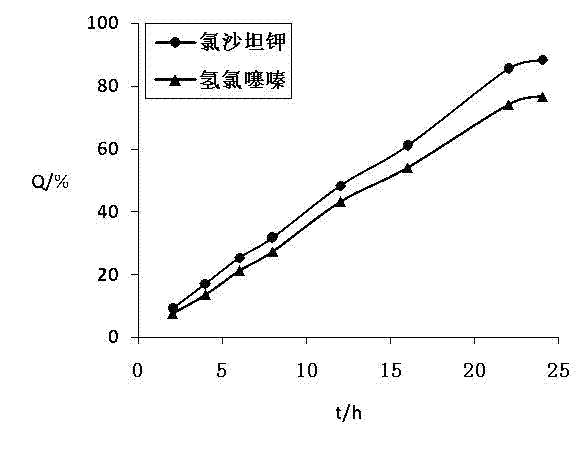
[0111] The test method of the release of the two active ingredients of the losartan potassium hydrochlorothiazide osmotic pump controlled-release tablet prepared by the present embodiment is the same as in Example 1, and the release curve recorded is as follows: figure 2 .
Embodiment 3
[0113] The hydrochlorothiazide-urea solid dispersion in Example 1 is replaced with hydrochlorothiazide-hydroxypropyl-β-cyclodextrin inclusion compound, and its addition amount is 200mg, the hydrochlorothiazide-hydroxypropyl-β-cyclodextrin inclusion compound The preparation method is:
[0114] Weigh hydrochlorothiazide, place it in a container, add 0.1mol / LNaOH, stir electromagnetically until hydrochlorothiazide dissolves, add hydroxypropyl-β-cyclodextrin, the molar ratio of added hydroxypropyl-β-cyclodextrin to hydrochlorothiazide is 3:1, add 0.1mol / L HCL drop by drop under electromagnetic stirring at 38°C, adjust the pH value to 5-7, rotate the water in the above solution at 70°C, and vacuum dry at 60°C to obtain Hydrochlorothiazide-hydroxypropyl-β-cyclodextrin inclusion compound solid dispersion.
[0115] Others are the same as in Example 1.
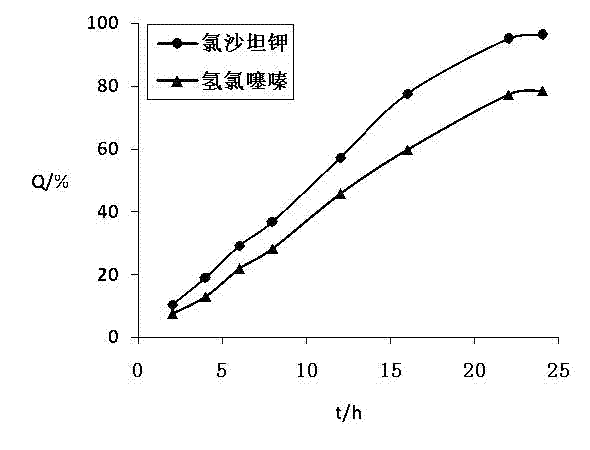
[0116] The test method of the release of the two active ingredients of the losartan potassium hydrochlorothiazide osmotic pump cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com