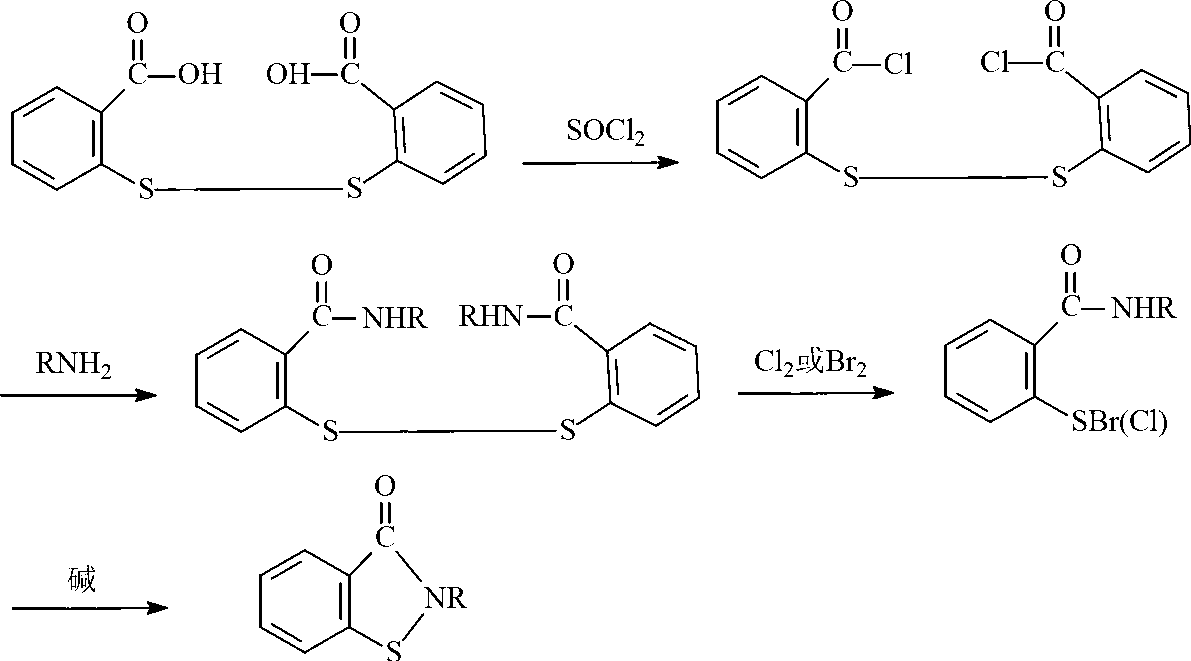
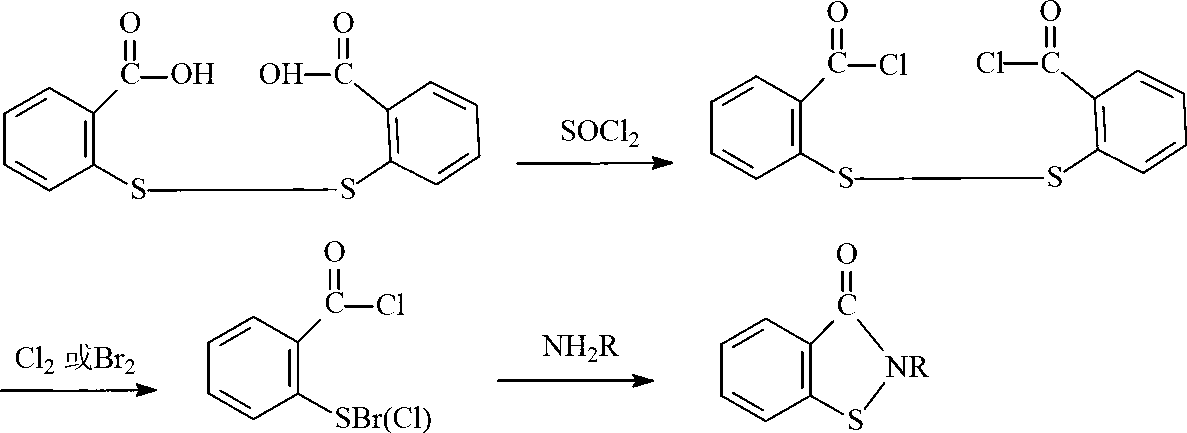
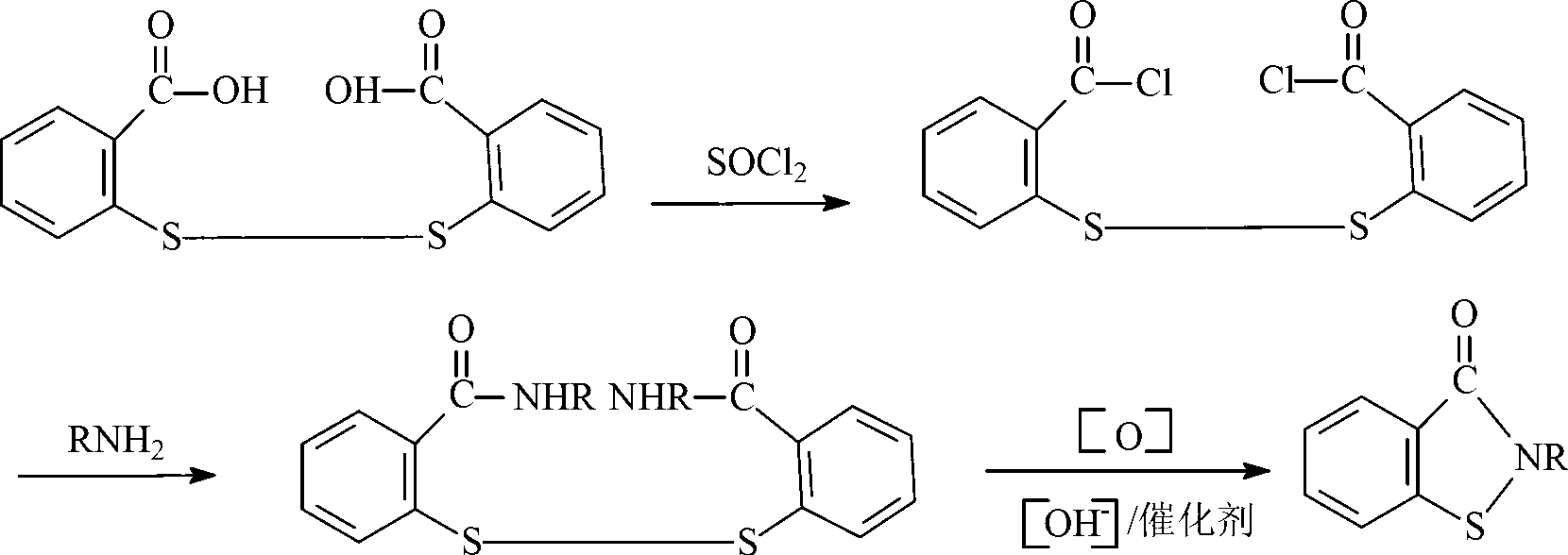
Preparation method of N-butyl-1,2-benzo isothiazolin-3-ketone
A technology of benzisothiazoline and n-butyl, applied in the field of preparation of N-n-butyl-1,2-benzisothiazolin-3-one, can solve the problem of difficult to meet environmental protection requirements and incomplete halogen absorption , serious equipment corrosion and other problems, to achieve the effect of low production cost, high product yield, and reduce the pressure of environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Synthesis of N-n-butyl-2,2'-dibenzamide disulfide
[0025] Add 34.3g of 2,2-dibenzoyl chloride disulfide and 100mL of toluene into the flask, add 14.6g of n-butylamine dropwise within 30°C, keep warm for 1 hour after the dropwise addition, and stop the reaction. Suction filtration, the product was washed several times with water and ethanol, and dried to obtain milky white N-n-butyl-2,2'-dibenzamide powder.
[0026] (2) Synthesis of N-n-butyl-1,2-benzisothiazolin-3-one
[0027] Add 41.7g of N-n-butyl-2,2'-dibenzamide disulfide, 100mL of toluene, aqueous sodium hydroxide solution (8.0g of sodium hydroxide, 200mL of water) and 20.2g of triethylamine into the reaction flask, and heat up to Slowly add 40.8g of 10% hydrogen peroxide solution dropwise to the reaction system within 8 hours at 55°C. After the dropwise addition, distill off triethylamine and toluene, pour the remaining liquid into a 500mL beaker, separate the lower organic phase, and dry to obtain yellow N ...
Embodiment 2
[0029] (1) Synthesis of N-n-butyl-2,2'-dibenzamide disulfide
[0030] The synthesis process is the same as step (1) of Example 1.
[0031] (2) Synthesis of N-n-butyl-1,2-benzisothiazolin-3-one
[0032] Add 41.7g of N-n-butyl-2,2'-dibenzamide disulfide, 100mL of toluene, aqueous sodium hydroxide solution (8.0g of sodium hydroxide, 200mL of water) and 30.4g of triethylamine into the reaction flask, and heat up to 60°C, slowly add 10% hydrogen peroxide solution dropwise to the reaction system within 6 hours, after the drop is completed, lower the temperature to 50°C and continue the reaction for 1 hour to end the reaction. Evaporate triethylamine and toluene, separate the lower organic phase, and dry to obtain yellow N-n-butyl-1,2-benzisothiazolin-3-one liquid with a yield of 32.4%.
Embodiment 3
[0034] (1) Synthesis of N-n-butyl-2,2'-dibenzamide disulfide
[0035] The synthesis process is the same as step (1) of Example 1.
[0036] (2) Synthesis of N-n-butyl-1,2-benzisothiazolin-3-one
[0037] Add 41.7g of N-n-butyl-2,2'-dibenzamide disulfide, 50mL of toluene, aqueous sodium hydroxide solution (16.0g of sodium hydroxide, 200mL of water) and 40.5g of triethylamine into the reaction flask, and raise the temperature to At 55°C, slowly add 51.0 g of 10% hydrogen peroxide solution dropwise into the reaction system within 6 hours, keep the temperature for 1 hour after the drop, and finish the reaction. Evaporate triethylamine and toluene, pour the remaining liquid into a 500mL beaker, separate the lower organic phase, and dry to obtain a yellow N-n-butyl-1,2-benzisothiazolin-3-one liquid with a yield of 28.1 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com