2-acetylpyridine benzoyl hydrazine molybdenum complex and preparation method thereof
A technology of acetylpyridinebenzohydrazide and molybdenum complex, which is applied in the field of 2-acetylpyridinebenzohydrazide molybdenum complex and its preparation, and can solve the problem of molybdenum Schiff base complex, etc. problems, to achieve excellent catalytic performance, environmental friendliness, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Weigh 10 mmol of benzoyl hydrazide, put it into a round bottom flask (50 ml) containing 20 ml of absolute ethanol, heat and stir at 50°C, add 10 mmol of 2-acetylpyridine after the benzoyl hydrazide is completely dissolved, After stirring for 1 hour, it was allowed to stand, and white needle-like crystals were precipitated after 7 days. Suction filtration, washing with a small amount of ethanol to obtain 2-acetylpyridine benzohydrazide Schiff base complex.
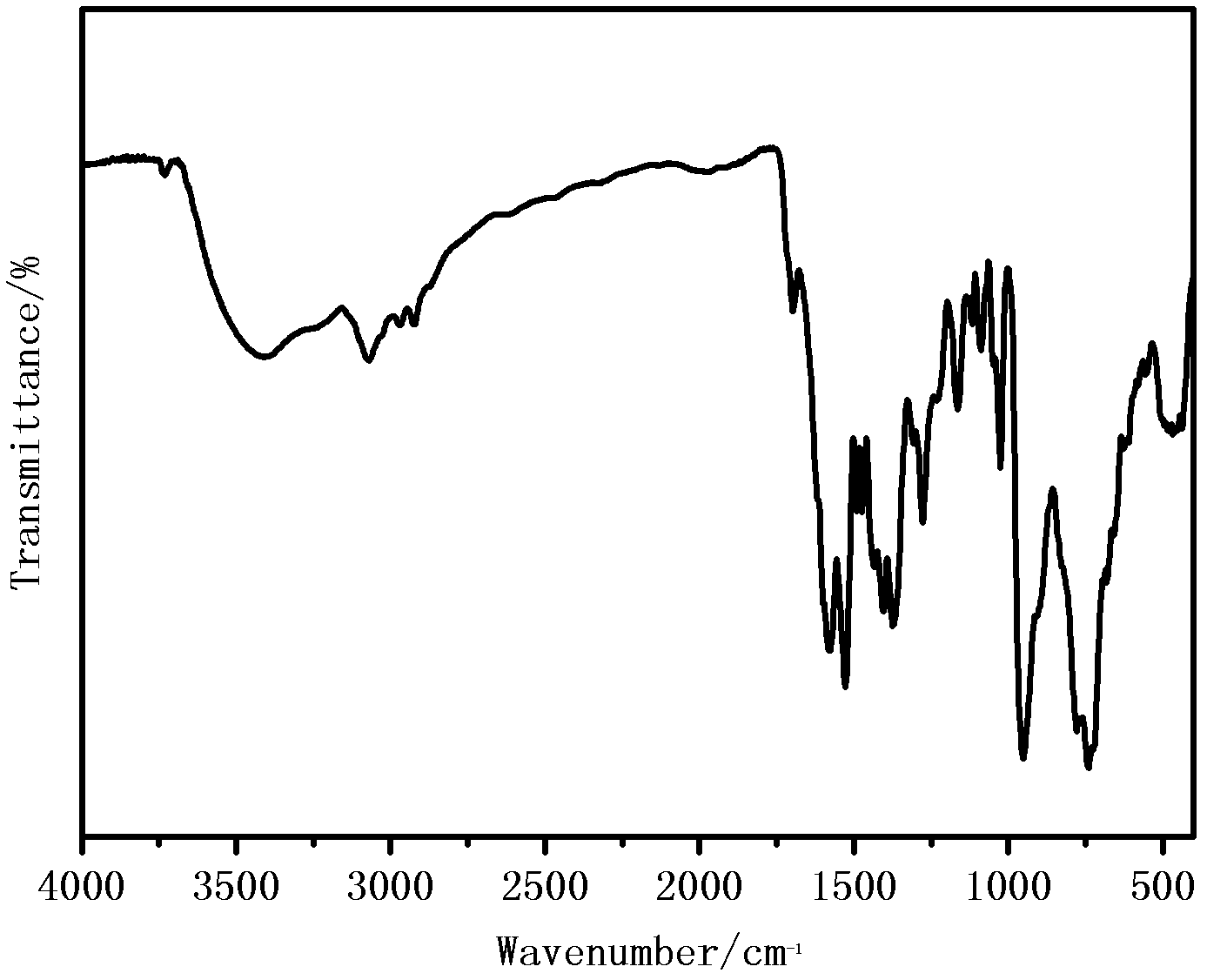
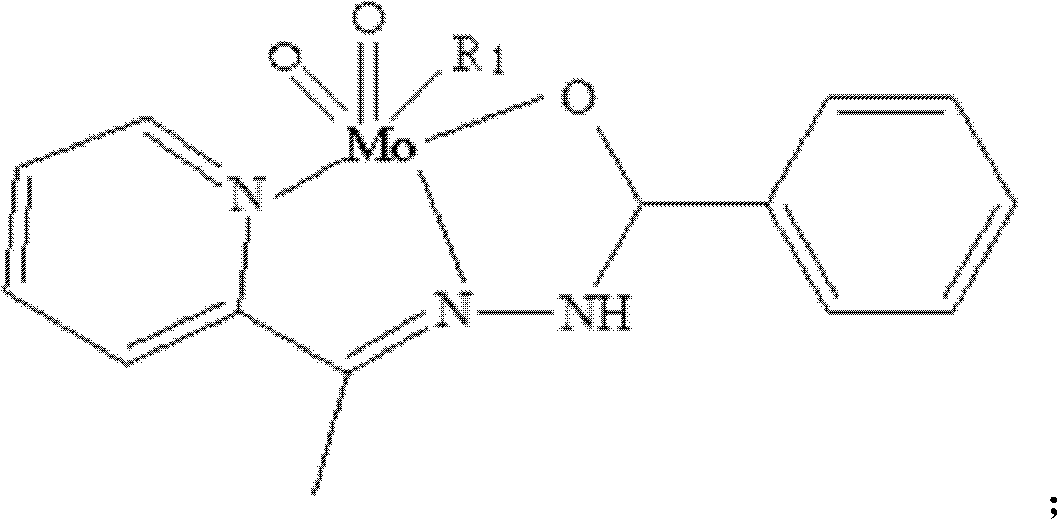
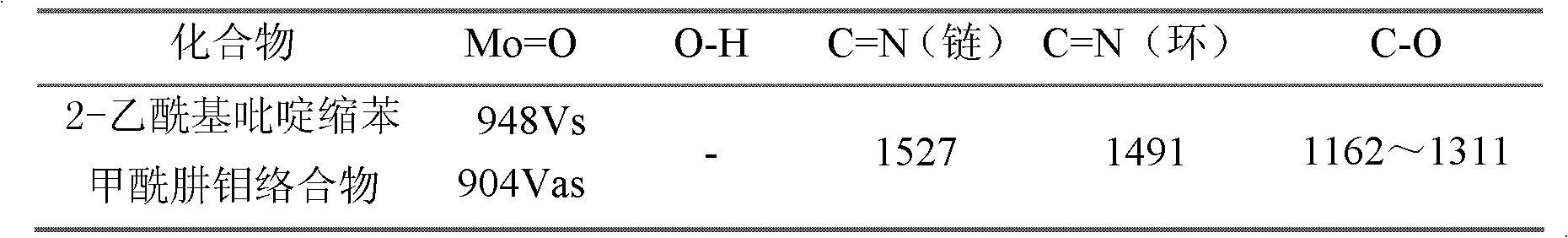
[0019] Weigh 5mmol of 2-acetylpyridine benzoyl hydrazide Schiff base complex, put it into a round bottom flask (50ml) containing 15ml of absolute ethanol, heat and stir at 75℃, wait until 2-acetylpyridine benzoyl hydrazide After the hydrazine is completely dissolved, 5mmol molybdenum acetylacetonate is added, and orange powder is precipitated. Continue heating and stirring for 2 hours and then suction and filter. After washing with ethanol and drying under vacuum at 40℃, an orange powder product is obtained. Its structu...
Embodiment 2
[0022] Weigh 10 millimoles of benzoyl hydrazide and put it into a round bottom flask (50 ml) containing 20 milliliters of pyridine. Heat and stir at 50°C. After the benzoyl hydrazide is completely dissolved, add 10 millimoles of 2-acetylpyridine and stir for 1 After a few hours, let it stand still, and after 7 days, white needle-like crystals were precipitated. Suction filtration, washing with a small amount of ethanol to obtain 2-acetylpyridine benzohydrazide Schiff base complex.
[0023] Weigh 5mmol of 2-acetylpyridine benzohydrazide Schiff base complex, put it into a round bottom flask (50ml) containing 15ml pyridine, heat and stir at 75℃, wait until 2-acetylpyridine benzohydrazide is complete After dissolving, add 5mmol molybdenum acetylacetonate, orange powder is precipitated, continue heating and stirring for 2 hours, suction filtration, pyridine washing and vacuum drying at 40℃ to obtain the product. Its structural formula is as follows, and its infrared analysis spectrum ...
Embodiment 3
[0026] Take tert-butyl hydroperoxide, n-propanol, and [Example 1] the synthesized 2-acetylpyridine benzoyl hydrazide molybdenum complex into the autoclave, seal the reactor and start heating and stirring. When it reaches the preset 100°C, the high-pressure pump pumps propylene, and the amount of propylene is determined by the pumped volume. The reaction time, after reaching 2 hours, remove the reaction kettle, cool to 10-15°C with ice water, and take the liquid phase into the frozen sampler. Continue to freeze the sampler at minus 20°C for 30 minutes and then open the gas valve, slowly release the propylene in the sampler, open the sampler, quickly transfer the remaining liquid to the chromatographic sample bottle and seal it for chromatographic analysis.
[0027] Reaction conditions: reaction temperature 100℃, reaction time 2 hours, rotation speed 540rpm, tert-butyl hydroperoxide (TBHP) dosage 70 mmol, n-propanol dosage 50 mmol, catalyst dosage 0.1 mmol, 60 ml reaction To the k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com