Caffeoyl-alpha-neoendorphin peptide derivative and use thereof as an anti-itch and anti-atopic material
A technology of peptide derivatives and compositions, applied in the field of caffeoyl endorphin derivatives, can solve the problems of easy oxidative deterioration and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
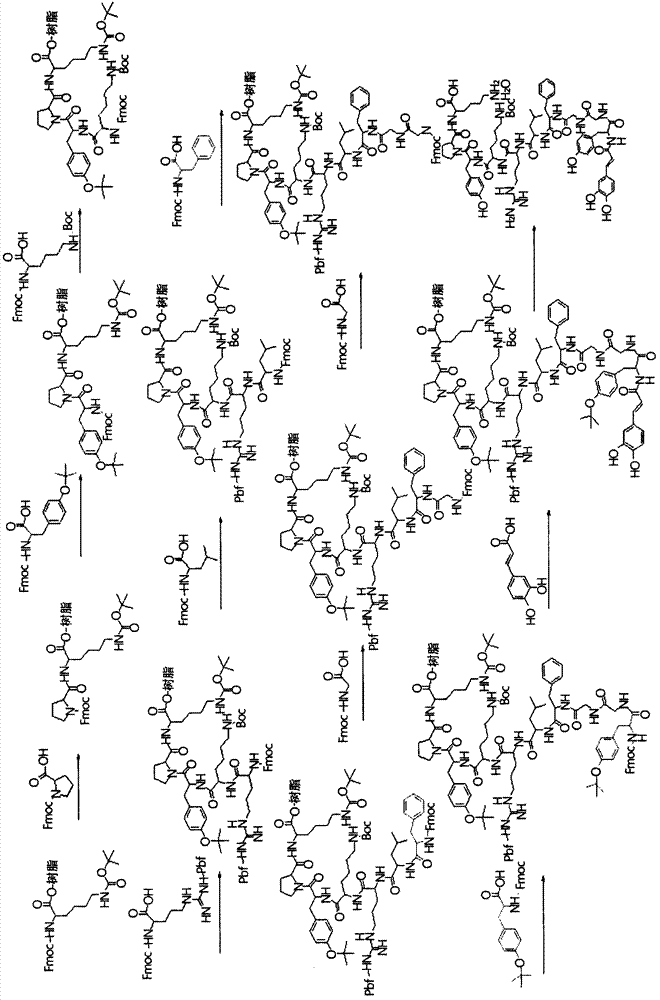
[0101] Embodiment 1: Caffeoyl (R 1 , R 2 and R 5 is hydrogen, and R 3 for R 4 Preparation of hydroxy)-neoendorphin derivatives
[0102] 1.1: NH 2 Synthesis of protected peptide resins
[0103] In general, peptides are synthesized using 9-fluorenylmethoxycarbonyl (Fmoc) as an amino acid protecting group using the usual solid phase peptide synthesis (SPPS), and N-hydroxybenzotri Azole (N-hydroxybenzotriazole, HOBt) and N, N'-dicyclohexylcarbodiimide (N, N'-dicyclohexylcarbodiimide, DCC) as active agents to extend amino acid residues [References: Wang C.Chan, Perter D. White, 'Fmoc solid phase peptide synthesis', Oxford].
[0104] 1.2: Synthesis of caffeoyl-α-neoendorphin derivatives
[0105] To the NH synthesized by the above method 2 Protected peptide (-20% piperidine / NMP solution was added to the resin to remove the Fmoc bound to the amino group, and N-methyl-2-pyrrolidone (N-methyl-2-pyrrolidone, NMP) and dichloromethane (dichloromethane, DCM) after washing, at room...
experiment example 1
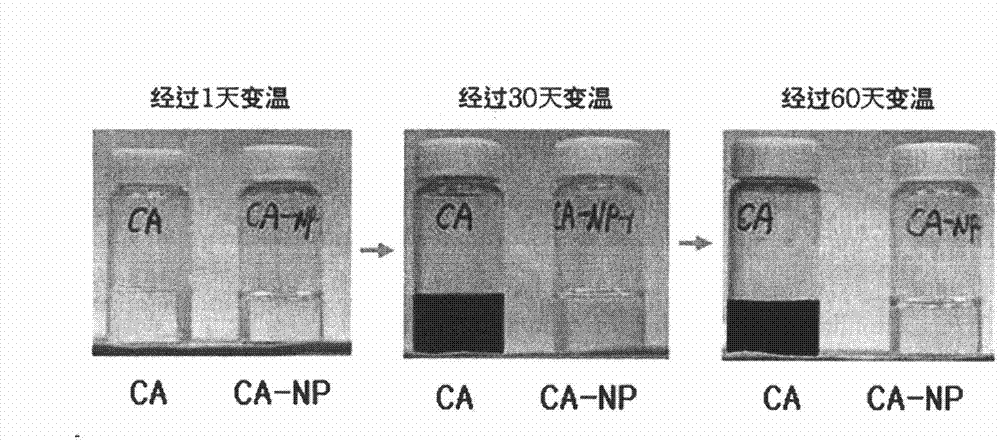
[0109] Experimental Example 1: Physical Stability Test of Caffeoyl-α-Neoendorphin Derivatives During Variable Temperature Treatment
[0110] In the case of cosmetics, considering the characteristics of products that are easily exposed to various external environments, physical stability under severe variable temperature conditions is an essential condition. The caffeoyl-α-neoendorphin derivative prepared in Example 1 has improved oxidation stability compared with caffeic acid in an aqueous solution state, and in order to confirm this improved physical stability After a certain period of storage under variable temperature conditions, a test to measure the degree of decomposition is carried out.
[0111] First, an aqueous solution of caffeoyl-α-neoendorphin derivatives at a concentration of 10,000ppm was subjected to variable temperature treatment conditions repeated in units of 8 hours at low temperature (4°C), normal temperature (20°C) and high temperature (40°C). , at interv...
experiment example 2
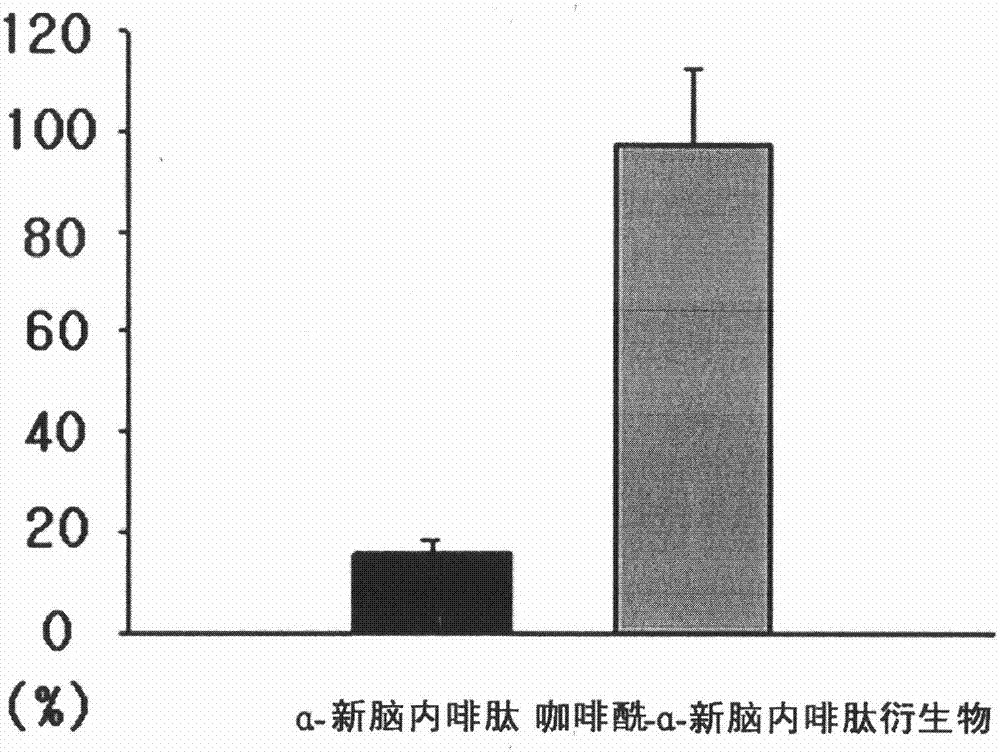
[0114] Experimental Example 2: Peptidase Stability Test of Caffeoyl-α-Neoendorphin Derivatives
[0115] In the caffeoyl-α-neoendorphin derivatives prepared in Example 1, the main component for improving atopic dermatitis is the caffeic acid part, which plays an anti-allergic effect. At the same time, as the peptide part α-Neoendorphins effectively relieve pruritus in atopic dermatitis. However, α-neoendorphin, the precursor substance of the peptide part, is a structure of amino acids, and when it acts on the skin, it is very fragile to the action of proteolysis or peptidase. Therefore, it is used as a cosmetic When used as a raw material, there are certain limitations in its biological stability.
[0116] The caffeoyl peptide derivative of the present invention greatly improves its biological stability through the synthesis with caffeic acid, and in order to confirm the improved stability to peptidase hydrolase through the synthesis with caffeic acid, under the temperature co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com