Method for obtaining borazane, suitable for obtaining highly pure and very highly pure borazane
A kind of borane ammonia, non-solvent technology, applied in the field of obtaining borane ammonia containing low level, even very low level impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0073] a) Synthesis and removal of solids from the reaction medium
[0074] 288 g of ammonium carbonate and 4 liters of dry tetrahydrofuran (0.0005% in water) were introduced into a 4 liter reactor equipped with a stirring device and a condenser and placed under argon.
[0075] 112 g of sodium borohydride, which had been pretreated in an oven at 140° C. for 2 h, were introduced into this mixture.
[0076] The medium was stirred at 40 °C for 12 h.
[0077] Thereafter, the medium was filtered to remove unconsumed reagent and sodium carbonate formed.
[0078] b) Solvent evaporated
[0079] The obtained ammonium borane solution in tetrahydrofuran was introduced again into the reactor. The solvent (THF) was evaporated and the borane ammonia (product B) was subsequently recovered.
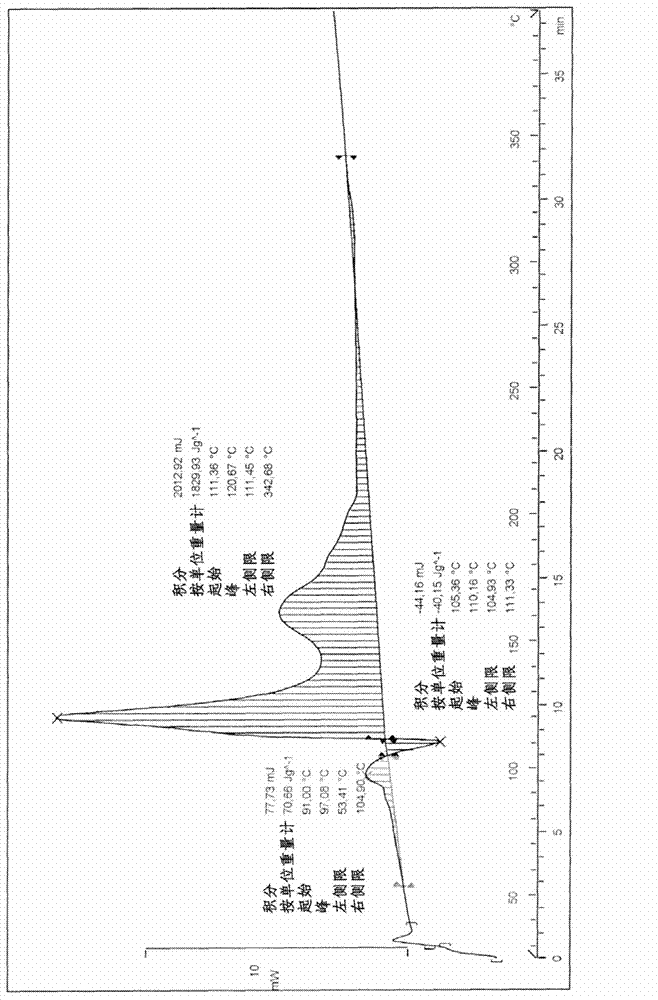
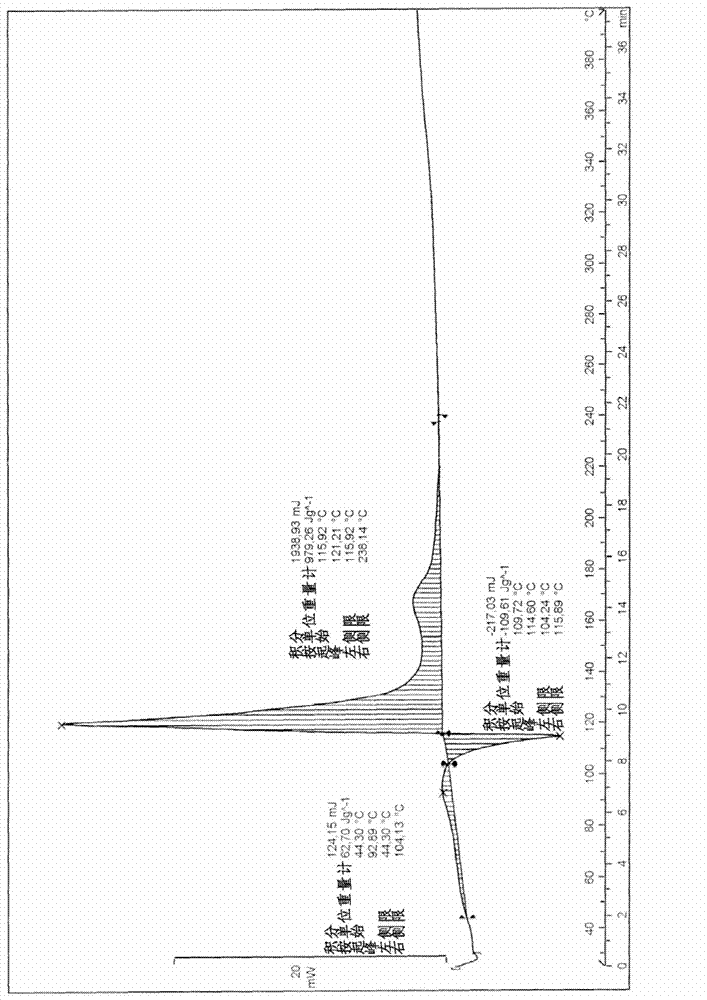
[0080] The thermogram of the borane ammonia is shown in Figure 1B. It involves exothermic decomposition in the solid state at low temperature (44°C). Its characteristics are described in Table 1 ab...
Embodiment II
[0083] a) Synthesis and removal of solids from the reaction medium
[0084] Step a) of Example I above was repeated.
[0085] b) Extraction with ether
[0086] The crude ammonium borane solid recovered after distilling off the THF was introduced into the Soxhlet extractor. Extraction was carried out by refluxing diethyl ether according to the teaching of application WO2007 / 106459. Extraction was performed at 35°C for 12h.
[0087] c) Solvent evaporated
[0088] After extraction, the ether was evaporated, and the borane ammonia was recovered and dried, and finally stored under an argon atmosphere.
[0089] The thermogram of the extracted ammonia borane (product C) is shown in Figure 1C. It includes exothermic decomposition in the solid state at a higher temperature than the exothermic decomposition of products A and B. Its characteristics are described in Table 1 above.
Embodiment 1
[0091] a) Synthesis and removal of solids from the reaction medium
[0092] Step a) of Example I above was repeated.
[0093] b) Purification according to the invention
[0094] The ammonium borane solution in tetrahydrofuran was introduced again into the reactor, followed by partial evaporation until 400 ml remained at the bottom of the vessel, ie 10% of the total amount of THF introduced at the beginning of the synthesis.
[0095] Then 2.5 liters of dry dichloromethane (0.005% water) were introduced into the reactor under vigorous stirring. Ammonia borane precipitated, which was then filtered and dried in an oven at 20° C. under vacuum for 12 h.
[0096] 70 g of borazane powder was recovered. Therefore, the yield of this method is greater than 70%.
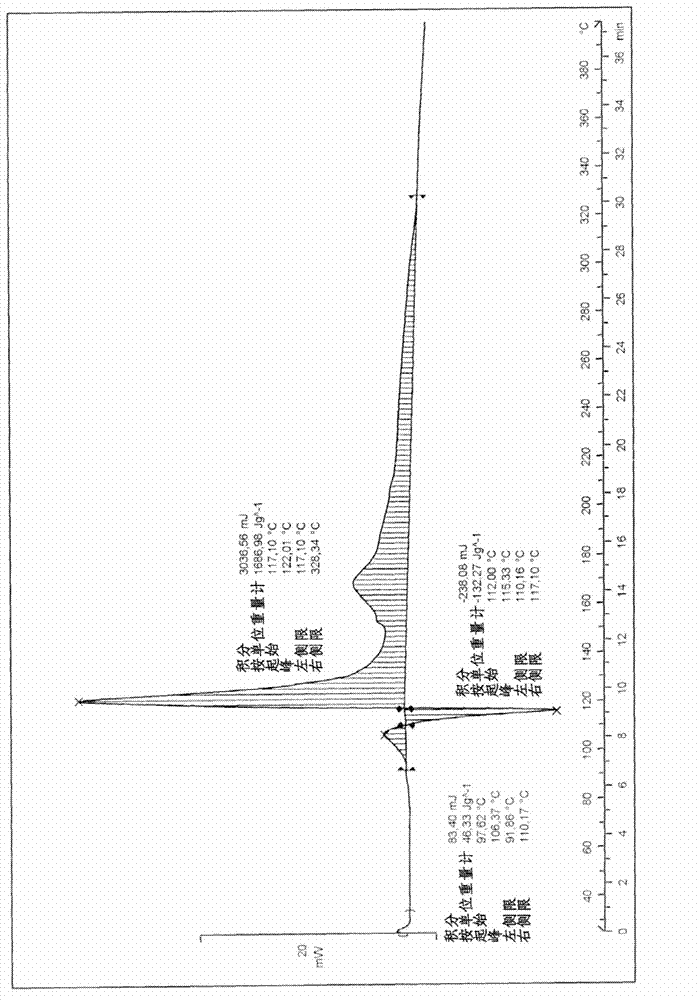
[0097] The thermogram of described powder is shown in appendix Figure 2A , which includes exothermic decomposition in the solid state at a higher temperature than the exothermic decomposition of products A and B. Its char...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com