Solid acid catalyst for 5-hydroxymethyl furfural synthesis and preparation method thereof
A solid acid catalyst, the technology of hydroxymethyl furfural, applied in the direction of organic chemistry and the like, can solve the problems of not reaching large-scale production, low catalytic activity, polluting the environment, etc., and achieve safe and reliable production process, simple preparation process, and high catalytic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
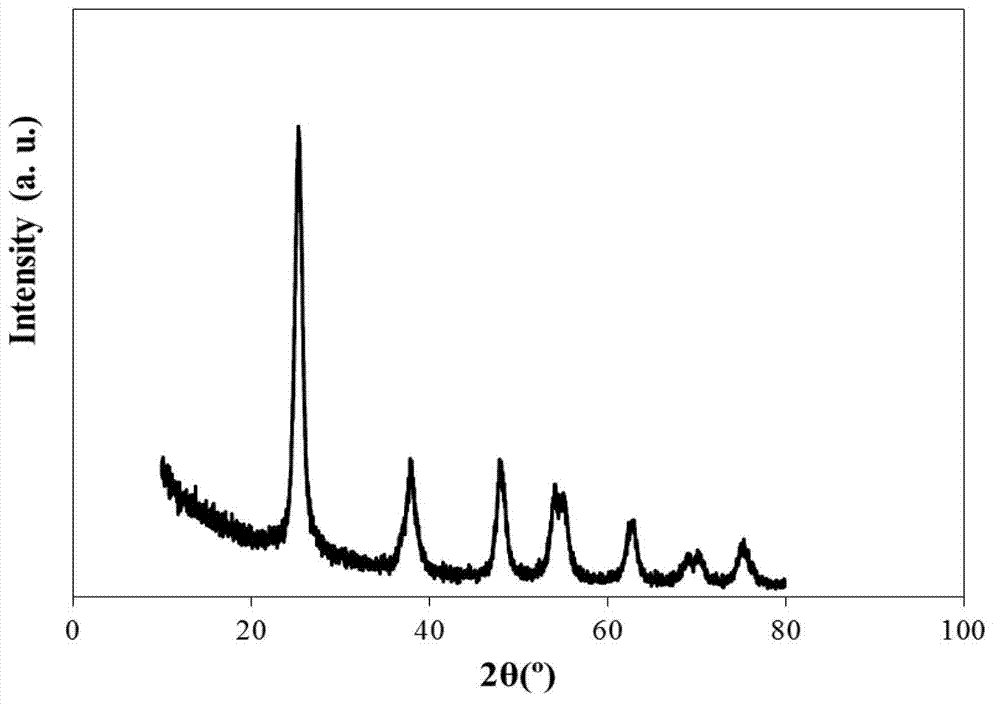
[0028] Weigh 18.9690g TiCl 4 , Dilute with 100mL deionized water until no white smoke is emitted, then stir and titrate with ammonia water to pH=10 to complete the precipitation. Stand at room temperature and age for 24 hours, and then wash the precipitate with deionized water until there is no chloride ion (using AgNO 3 detection), and the precipitate was dried at 110°C for 12h. Grind the dried sample into powder (about 100-120 mesh), add 0.5mol / L H 2 SO 4 solution, stirred and impregnated at 600rpm for 6h, filtered, and dried at 110°C for 12h, the obtained SO 4 2- / TiO 2 Place in a muffle furnace and bake at 600°C for 4h.
Embodiment 2
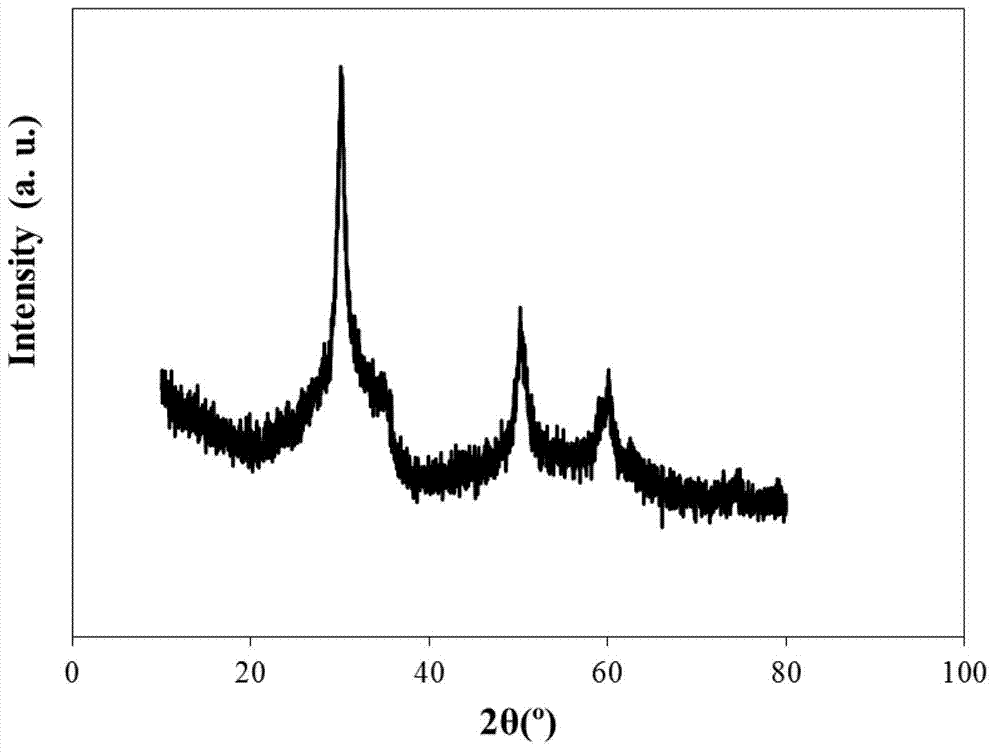
[0030] Weigh 32.2250g ZrOCl 2 ·8H 2 O, dissolved in 100mL deionized water, then stirred and titrated with ammonia water to pH=10 to complete the precipitation. Stand at room temperature and age for 24 hours, and wash the precipitate with deionized water until there is no chloride ion (using AgNO 3 detection), and the precipitate was dried at 110°C for 12h. Grind the dried sample into powder (about 100-120 mesh), add 0.5mol / L H 2 SO 4solution, stirred and impregnated at 600rpm for 6h, filtered, and dried at 110°C for 12h, the obtained SO 4 2- / ZrO 2 Place in a muffle furnace and bake at 600°C for 4h.
Embodiment 3
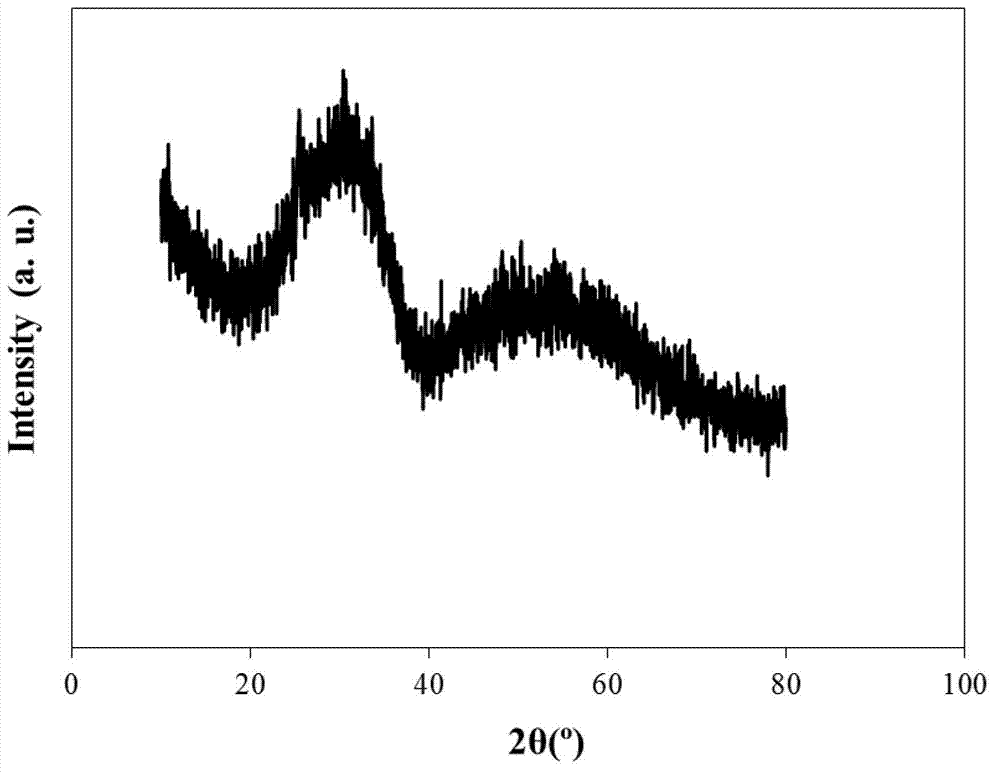
[0032] Weigh 9.4845g TiCl 4 and 16.1125 g ZrOCl 2 ·8H 2 O, dissolved in 100mL deionized water, then stirred and titrated with ammonia water to pH=10 to complete the precipitation. Stand at room temperature and age for 24 hours, and then wash the precipitate with deionized water until there is no chloride ion (using AgNO 3 detection), and the precipitate was dried at 110°C for 24h. Grind the dried sample into powder (about 100-120 mesh), add 0.5mol / L H 2 SO 4 , impregnated with stirring at 600rpm for 4h, filtered, and dried at 110°C for 12h, the obtained SO 4 2- / TiO 2 -ZrO 2 Place in a muffle furnace and bake at 550°C for 4h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com