Method for preparing 9-(4-sulfophenyl) octadecanoic acid or 10-(4-sulfophenyl) octadecanoic acid
A technology of phenyl octadecanoic acid and octadecanoic acid, which is applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of acidity reduction, expensive chlorosulfonic acid, unfavorable reaction, etc., and achieve complete reaction, strong sulfonation ability, single structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
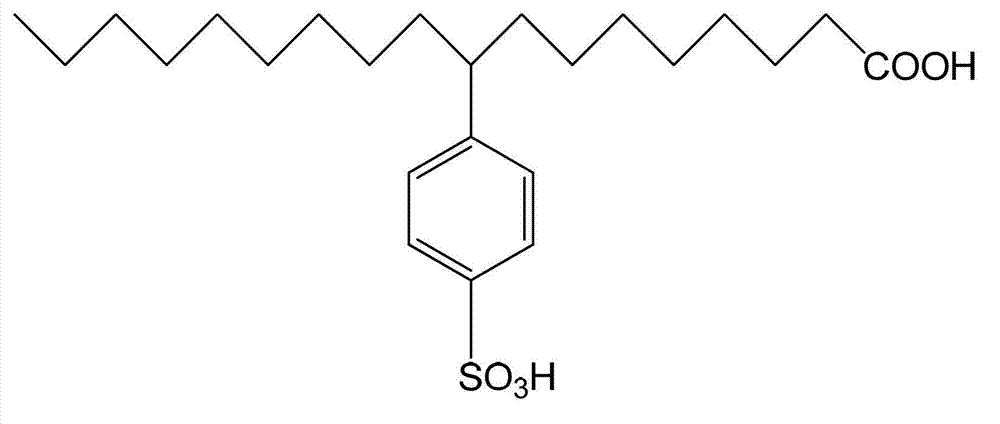
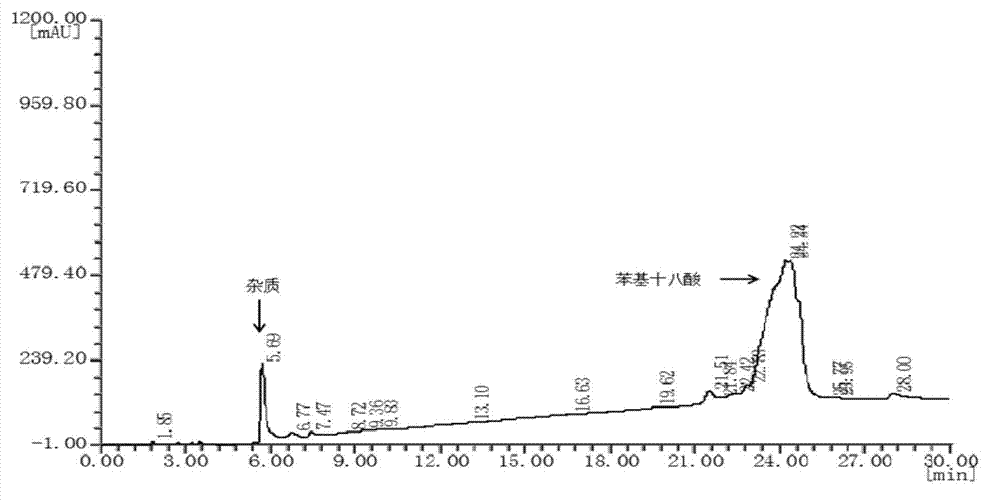
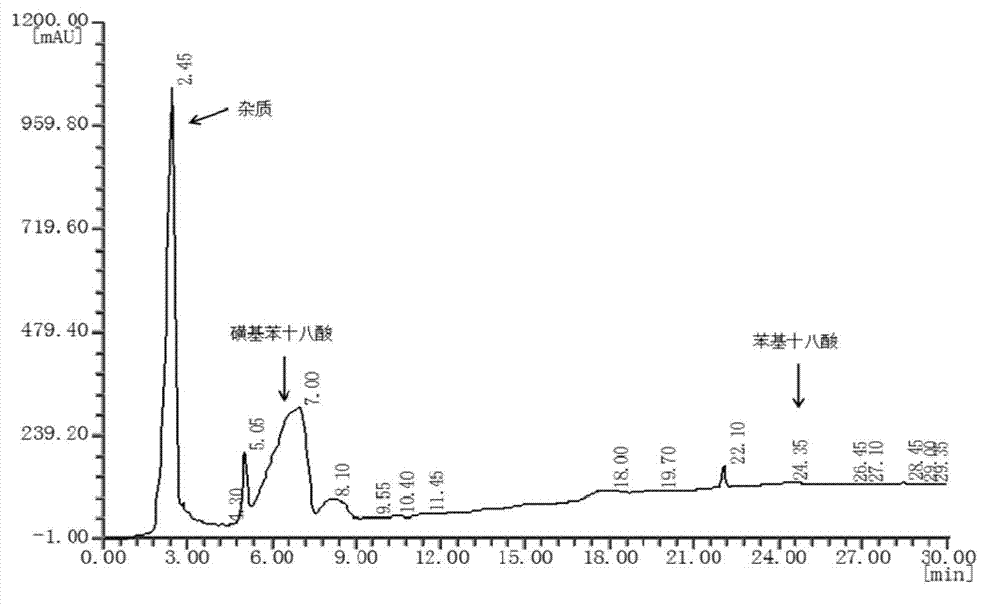
[0028] In 50 mL of dichloromethane organic solvent solution with a concentration of 0.2 mol / L 9-phenyloctadecanoic acid, SO 3 Gas 0.01mol (molar ratio 1:1). Reaction at 20°C for 2h. After the reaction, the obtained reaction solution was rotary evaporated to remove the solvent, and extracted with a mixed solvent of ethanol and water to obtain 9-(4-sulfobenzene)octadecanoic acid with a yield of 90.36%. The molecular structure of 9-(4-sulfophenyl) octadecanoic acid is as follows figure 1 shown.
Embodiment 2
[0030] In 50 mL of chloroform organic solvent solution with a concentration of 0.2 mol / L10-phenyloctadecanoic acid, SO 3 Gas 0.015mol (molar ratio 1:1.5). Reaction at 20°C for 4h. After the reaction, the obtained reaction solution was rotary evaporated to remove the solvent, and extracted with a mixed solvent of methanol and water to obtain 10-(4-sulfobenzene)octadecanoic acid with a yield of 91.77%.
Embodiment 3
[0032] In 50 mL concentration of 0.2 mol / L 9-phenyl octadecanoic acid chloroform organic solvent solution, feed SO3 gas 0.02 mol (molar ratio 1: 2) while stirring in an ice-salt bath. Reaction at 20°C for 6h. After the reaction, the resulting reaction solution was rotatably evaporated to remove the solvent, and extracted with a mixed solvent of methanol and water to obtain 9 or 10-(4-sulfobenzene)octadecanoic acid with a yield of 92.68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com