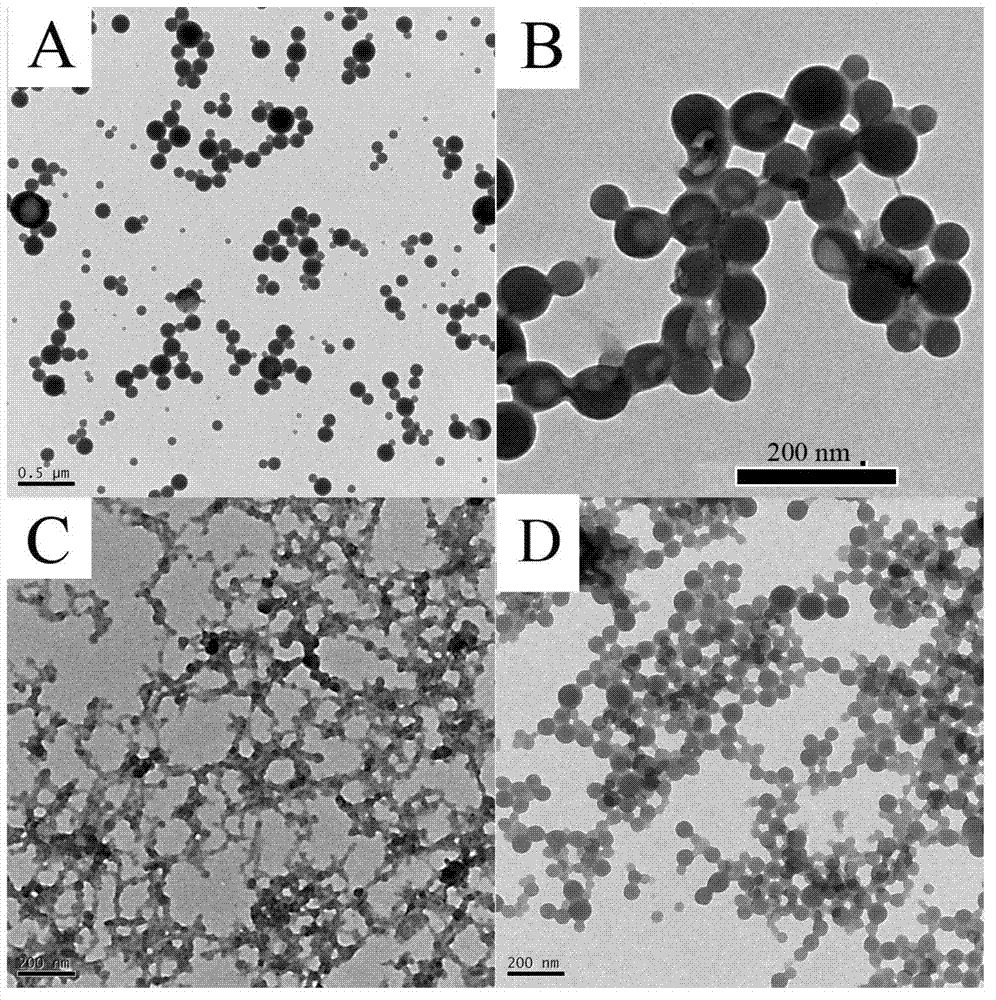
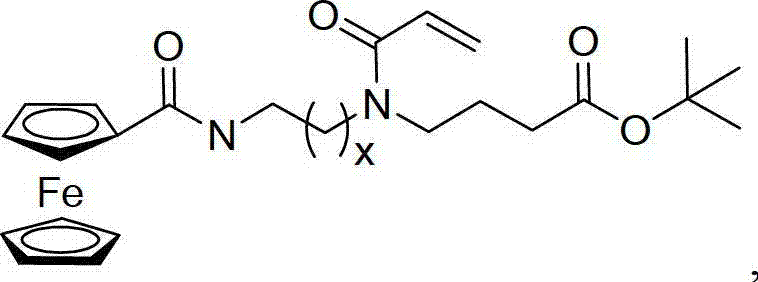
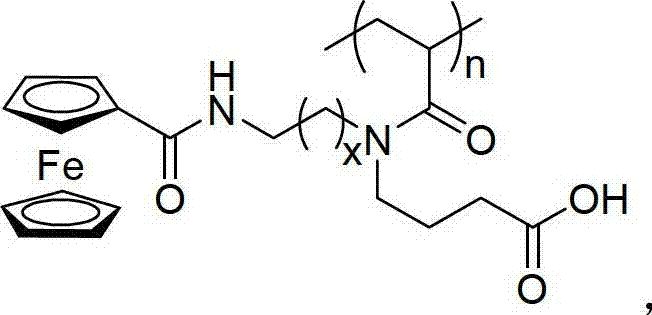
Acrylamide type monomer containing dicyclopentadienyl iron structure, as well as amphipathic polymer, preparation method and use thereof
A technology of amphiphilic polymers and acrylamide, which is applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve the problems of complex synthesis steps, difficult synthesis, and lack of universality of amphiphilic block copolymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthesis of embodiment 1. compound 2a
[0046]
[0047] Add ferrocenecarboxylic acid (9.2g, 40mmol), N-hydroxysuccinimide (5.75g, 50mmol), 200mL tetrahydrofuran into a dry 500mL three-necked flask, stir at room temperature for 5 minutes, then add DCC (10.3 g, 50mmol), stirred at room temperature for 12 hours, filtered to remove insoluble matter, slowly added the filtrate to 200mL tetrahydrofuran solution dissolved with triethylamine (5.6mL, 40mmol), ethylenediamine (5.4mL, 80mmol), and stirred overnight at room temperature. The insoluble matter was removed by filtration, concentrated, added dichloromethane, washed twice with water and saturated brine, dried over anhydrous sodium sulfate, and column chromatographed to obtain 8.31g of 2a, with a yield of 76.4%.
[0048] FT-IR (film): v (cm -1 ):3290,3081,2930,2856,1734,1627,1544,1453,1376,1301,1185,821.
[0049] 1 H NMR: δ(ppm): 1.85, 3.75, 4.12, 4.36, 4.74, 5.75.
Embodiment 2
[0050] The synthesis of embodiment 2 compound 2b
[0051]
[0052] Add ferrocenecarboxylic acid (9.2g, 40mmol), N-hydroxysuccinimide (5.75g, 50mmol), and 200mL dichloromethane into a dry 500mL three-necked flask, stir at room temperature for 5 minutes, and then add DCC to the solution (10.3g, 50mmol), stirred at room temperature for 12 hours, filtered to remove insoluble matter, and slowly added the filtrate to 200mL dichloromethane solution in which triethylamine (5.6mL, 40mmol) and propylenediamine (6.6mL, 80mmol) were dissolved, Stir overnight at room temperature. The insoluble matter was removed by filtration, concentrated, added dichloromethane, washed twice with water and saturated brine, dried over anhydrous sodium sulfate, and column chromatographed to obtain 9.17g of 2b with a yield of 80.2%.
[0053] FT-IR (film): v (cm -1 ):3292,3083,2931,2856,1735,1628,1544,1454,1378,1301,1186,821.
[0054] 1 H NMR: δ(ppm): 1.43, 1.72, 2.71, 4.12, 4.36, 4.74, 5.75.
Embodiment 3
[0055] The synthesis of embodiment 3 compound 2c
[0056]
[0057] Add ferrocenecarboxylic acid (9.2g, 40mmol), N-hydroxysuccinimide (5.75g, 50mmol), and 200mL dichloromethane into a dry 500mL three-necked flask, stir at room temperature for 5 minutes, and then add DCC to the solution (10.3g, 50mmol), stirred at room temperature for 12 hours, filtered to remove the insoluble matter, and slowly added the filtrate to 200mL of dichloromethane solution in which triethylamine (5.6mL, 40mmol) and butylenediamine (8.0mL, 80mmol) were dissolved, Stir overnight at room temperature. The insoluble matter was removed by filtration, concentrated, added dichloromethane, washed twice with water and saturated brine, dried over anhydrous sodium sulfate, and column chromatographed to obtain 8.68g of 2c with a yield of 72.3%.
[0058] FT-IR (film): v (cm -1 ):3282,3081,2930,2856,1734,1627,1539,1455,1372,1180,828.
[0059] 1 H NMR: δ(ppm): 1.48, 1.60, 2.71, 3.36, 4.20, 4.33, 4.70, 5.75.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com