Synthetic method of anethole
A synthesis method and technology of anethole, which is applied in the field of preparation of spice compounds, can solve the problems of long negative pressure continuous dehydration reaction time, high side reactions of acylation reaction, and reduced safety factor, so as to reduce purification costs, have few side reactions, The effect of energy consumption reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
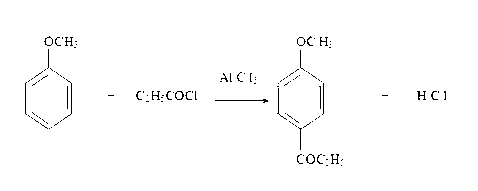
[0038] Under stirring state, drop into 118.8kg anisole and 133.5kgAlCl in the reactor 3 , lower the temperature to 5°C, add 92.5kg of propionyl chloride dropwise, control the temperature at 5°C to 10°C during the dropwise addition, and keep the temperature at 5°C to 10°C for 3 hours to complete the reaction, and neutralize with sodium hydroxide until neutral. The crude product of p-methoxypropiophenone was obtained, and the chromatographically effective amount was 148kg.
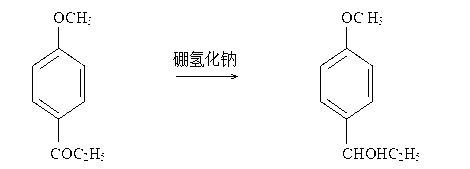
[0039] Add 14.8 kg of sodium borohydride to the above-mentioned crude product of p-methoxypropiophenone, control the temperature at 20°C to 30°C for the reduction reaction, end the reaction when the content of p-methoxypropiophenone is less than 0.5%, and neutralize with acetic acid to neutralize properties, the crude product of 1-(4-methoxyphenyl)-1-propanol was obtained, and the effective amount for chromatographic analysis was 147kg.
[0040] In above-mentioned 1-(4-methoxyphenyl)-1-propanol crude produc...
Embodiment 2
[0043] Under stirring state, drop into 129.6kg anisole and 133.5kgAlCl in the reactor 3 , lower the temperature to 5°C, add 92.5kg of propionyl chloride dropwise, control the temperature at 5°C to 10°C during the dropwise addition, and keep the temperature at 5°C to 10°C for 3 hours to complete the reaction, and neutralize with sodium hydroxide until neutral. The crude product of p-methoxypropiophenone was obtained, and the chromatographically effective amount was 149kg.
[0044] Put 22.4kg of sodium borohydride into the above-mentioned crude product of p-methoxypropiophenone, control the temperature at 20°C to 30°C for reduction reaction, end the reaction when the content of p-methoxypropiophenone is lower than 0.5%, and neutralize with acetic acid to neutralize properties, the crude product of 1-(4-methoxyphenyl)-1-propanol was obtained, and the effective amount for chromatographic analysis was 148kg.
[0045] In above-mentioned 1-(4-methoxyphenyl)-1-propanol crude product,...
Embodiment 3
[0048] Under stirring state, drop into 140.4kg anisole and 133.5kgAlCl in the reactor 3 , lower the temperature to 5°C, add 92.5kg of propionyl chloride dropwise, control the temperature at 5°C to 10°C during the dropwise addition, and keep the temperature at 5°C to 10°C for 3 hours to complete the reaction, and neutralize with sodium hydroxide until neutral. Obtain p-methoxypropiophenone crude product, the chromatographic analysis effective amount is 146kg.
[0049] Put 29.2kg of sodium borohydride into the crude p-methoxypropiophenone, control the temperature at 20°C to 30°C for reduction reaction, end the reaction when the content of p-methoxypropiophenone is less than 0.5%, neutralize with acetic acid to properties, the crude product of 1-(4-methoxyphenyl)-1-propanol was obtained, and the effective amount for chromatographic analysis was 145kg.
[0050] In above-mentioned 1-(4-methoxyphenyl)-1-propanol crude product, drop into 362.5kg methyl alcohol, 1.45kg p-toluenesulfoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com