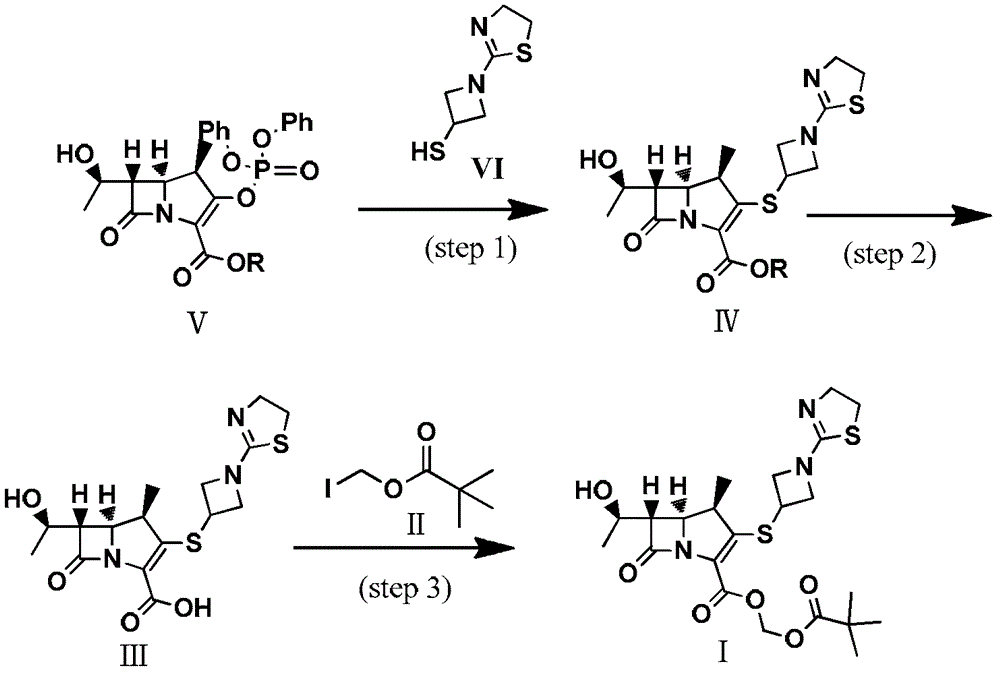
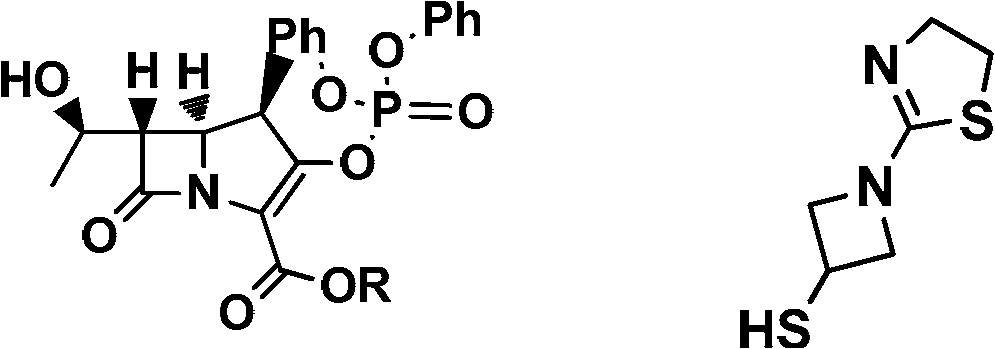Preparation method of tebipenem ester
A technology of tipipenem and southern acid, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high risk and high cost, and achieve the effect of simple operation, short route steps and saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, preparation of tipipenem acid and tipipenem piffate
[0038] Step 1: (1R,5S,6S)-1-Methyl--6-[(R)-1-Hydroxyethyl]-2-[1-(1,3-Dihydrothiazole)azetidinyl -3-] the preparation of thio-carbapenicillin-2-ene-3-carboxylate (formula IV)
[0039]
[0040] Compound (VI) (59.5g) (please indicate the formula) and compound (V) (25.2g) (R=Me) were dissolved in acetonitrile (180mL), acetone (180mL), N,N-dimethylformamide ( 18mL) in a mixed solvent, lower the temperature to 0°C, add diisopropylethylamine (41.8mL) dropwise, after dropping, control the temperature at 0°C, monitor the reaction with TLC until the reaction of the compound of formula (V) disappears, add water to quench the reaction, pump Filter, and the filter cake is washed with acetonitrile and water to obtain an off-white solid with a yield of 98%;
[0041] The structural confirmation data are as follows:
[0042] 1 H-NMRδ (600MHz CDCl3): 5.12-4.99 (2H, m), 4.79-4.73 (1H, m), 4.55-4.49 (4H, m), 4.37 (2...
Embodiment 2
[0054] Embodiment 2, preparation of tipipenem acid and tipipenem piffate
[0055] Step (1), (3) embodiment 1 simultaneously, just replace step (2) with following steps:
[0056] Compound (IV) (10g) was dissolved in a mixed solvent (volume ratio 1:2) of water and n-butanol (volume ratio 1:2) (120mL), adding (sodium dihydrogen phosphate) buffer solution to make the pH of the reaction system be 4.5. Stir at room temperature for 19 hours. After the reaction, add acetone to the aqueous phase for crystallization to obtain the compound of formula (III), tipipenem acid, with a yield of 66%.
[0057] The total yield of tipipenem pivoxil prepared by the method in Example 2 was 52.3%.
Embodiment 3
[0058] Example 3, preparation of tipipenem acid and tipipenem piffate
[0059] Step (1), (3) embodiment 1 simultaneously, just replace step (2) with following steps:
[0060] Compound (IV) (10g) was dissolved in a mixed solvent (120mL) of water and n-butanol with a volume ratio of 1:2, sodium dihydrogen phosphate-sodium hydroxide buffer solution was added to make the pH of the reaction system 11.5, and stirred at room temperature for 8 Hours, after the reaction was completed, acetone was added to the aqueous phase for crystallization to obtain the compound of formula (III), namely tipipenem acid, with a yield of 76%.
[0061] The total yield of tipipenem pivoxil prepared by the method in Example 3 was 60.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com