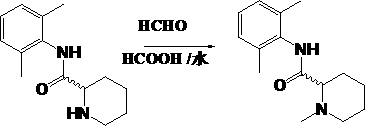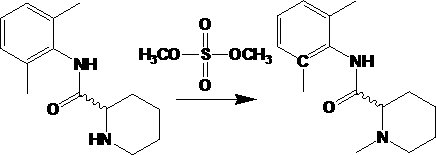Preparation method of mepivacaine and optical enantiomer of mepivacaine
A technology of mepivacaine and enantiomers, which is applied in the field of preparing mepivacaine and its optical antipodes, can solve the problems of quaternary ammonium salt by-products, expensive methyl iodide reagents, and high manufacturing costs, and achieve production costs Low cost, simple operation, less reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
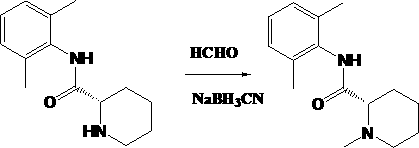
Method used
Image
Examples
Embodiment 1
[0024] Add 46g (1.0mol) of anhydrous formic acid to a 1-liter reaction flask, and add 46.46g (0.2 mol), stirred for 10 minutes, added 18g (0.6mol) of paraformaldehyde, heated in an oil bath to 90°C~95°C, and the tail gas absorption device showed gas generation. Heat preservation reaction for 8 hours. After the reaction, add 100ml (0.4mol) of 4N hydrochloric acid, evaporate the solvent to dryness under reduced pressure to obtain a light yellow slurry, dissolve it in 80ml of water, add 80ml of toluene to wash, separate the liquids, and control the temperature at 0°C~5°C in an ice bath , add 50ml of 18N sodium hydroxide to the water layer, extract with toluene 80ml×2, combine the organic phases, add 10g of anhydrous sodium sulfate and dry for 3 hours. After filtering, the filtrate was concentrated under reduced pressure with a rotary evaporator to obtain 45.1 g of mepivacaine. The yield is 91.7%. mp 149°C–151°C, MS (EI) C 15 h 22 N 2 O m / z (M +. ): 246.2.
Embodiment 2
[0026] Add 46g (1.0mol) of anhydrous formic acid to a 1-liter reaction flask, and add (S)-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide at a controlled temperature of 20°C to 25°C 46.46g (0.2mol), stir for 10 minutes, add 18g (0.6mol) of paraformaldehyde, heat the oil bath to 90°C~95°C, the tail gas absorption device shows gas generation. Heat preservation reaction for 8 hours. After the reaction, add 100ml (0.4mol) of 4N hydrochloric acid, evaporate the solvent to dryness under reduced pressure to obtain a light yellow slurry, dissolve it in 80ml of water, add 80ml of toluene to wash, separate the liquids, and control the temperature at 0°C~5°C in an ice bath , add 50ml of 18N sodium hydroxide to the water layer, extract with toluene 80ml×2, combine the organic phases, add 10g of anhydrous sodium sulfate and dry for 3 hours. After filtration, the filtrate was concentrated under reduced pressure with a rotary evaporator to obtain 45.3 g of the S-mepivacaine enantiomer. Yiel...
Embodiment 3
[0028] Add 46g (1.0mol) of anhydrous formic acid to a 1-liter reaction flask, and add (R)-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide at a controlled temperature of 20°C to 25°C 46.46g (0.2mol), stir for 10 minutes, add 18g (0.6mol) of paraformaldehyde, heat the oil bath to 90°C~95°C, the tail gas absorption device shows gas generation. Heat preservation reaction for 8 hours. After the reaction, add 100ml (0.4mol) of 4N hydrochloric acid, evaporate the solvent to dryness under reduced pressure to obtain a light yellow slurry, dissolve it in 80ml of water, add 80ml of toluene to wash, separate the liquids, and control the temperature at 0°C~5°C in an ice bath , add 50ml of 18N sodium hydroxide to the water layer, extract with toluene 80ml×2, combine the organic phases, add 10g of anhydrous sodium sulfate and dry for 3 hours. After filtration, the filtrate was concentrated under reduced pressure with a rotary evaporator to obtain 42.7 g of the thermal R-mepivacaine enantiome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com