Synthesis method of monoalkyl hydrocarbyl phosphonate
A technology of alkyl and cycloalkyl, which is applied in the field of synthesizing hydrocarbyl monoalkyl phosphate extractants, and can solve the problems of harsh reaction conditions, residues, and low product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] Synthetic methods include one or more of the following steps:
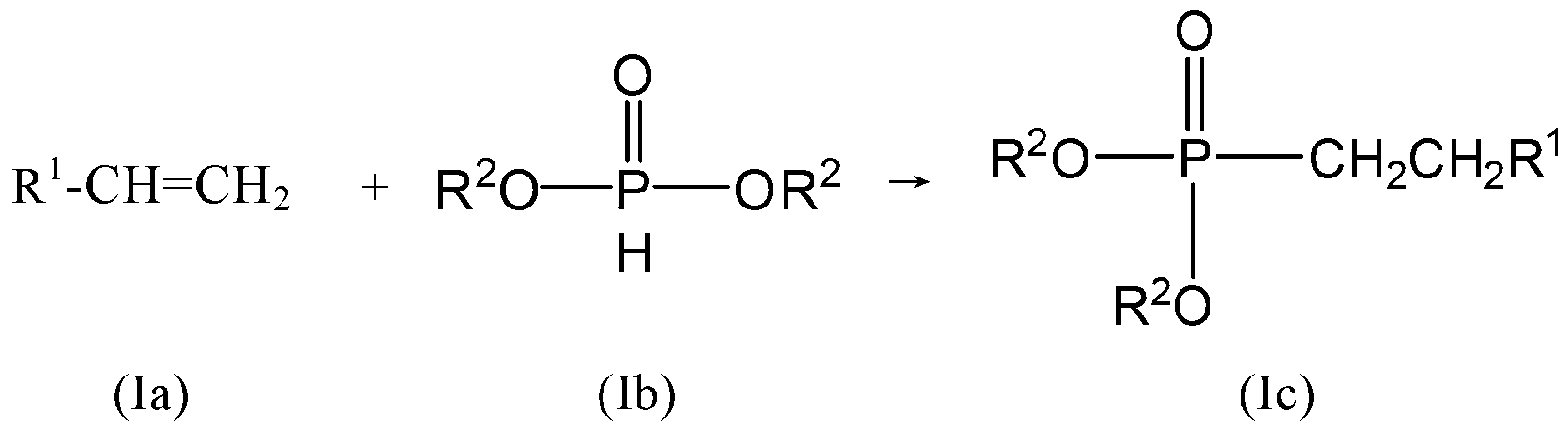
[0087] (a) in an inert solvent, the phosphonite diester is reacted with an alkene to obtain an alkylphosphonate diester; and / or
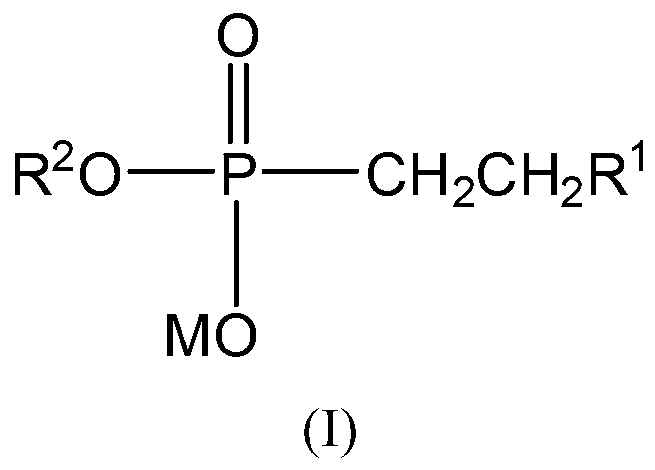
[0088] (b) in an inert solvent, under reflux conditions, the alkyl phosphonic acid diester obtained in step (a) is reacted with an alkali solution to obtain a compound of formula I;
[0089] In the above formulas, M and R 2 as defined above;
[0090] R 1 It is a substituted or unsubstituted C1-C18 alkyl group, a substituted or unsubstituted C3-C18 cycloalkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted C1-C3 alkylene aryl group;
[0091] or R 1 with substituted or unsubstituted -CH=CH 2 Constitute C3-C20 substituted and unsubstituted cycloalkenes together;
[0092] or R 1 with substituted or unsubstituted -CH 2 CH 2 - together form C3-C20 substituted or unsubstituted cycloalkanes;
[0093] Wherein, the definition of substitution is th...
Embodiment 1
[0121] Embodiment 1: the synthesis of 1-octylphosphonic acid monoisooctyl ester
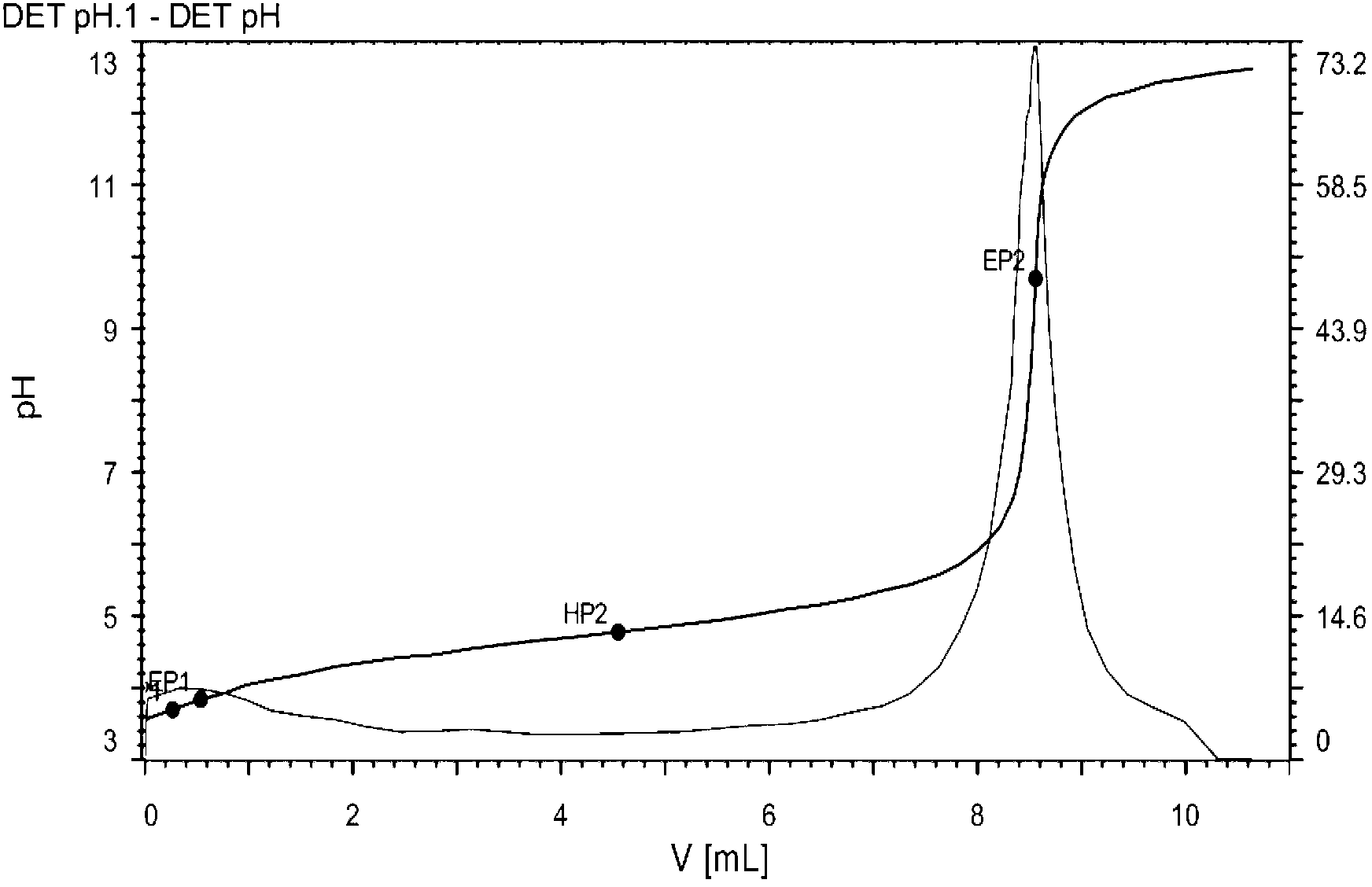
[0122] Add 10.6 g of sodium phosphinate monohydrate, 80 mL of glacial acetic acid, 11.2 g of 1-octene, and 4 mL of tert-butyl peroxybenzoate into a three-necked flask in sequence, heat to 130 degrees under normal pressure, and reflux for 2 hours. After adding 4 mL of initiator tert-butyl peroxybenzoate, the reflux reaction was continued for 2 hours. Wash twice with water, once with saturated brine, and extract twice with ether. Combine the organic phases, wash with water, dry over anhydrous sodium sulfate, filter with suction, and remove the solvent under reduced pressure to obtain 30.6 g of the product with a yield of 73.2%. Add 52ml of ethanolamine, heat and reflux for 30 hours, recover the excess ethanolamine under reduced pressure, dissolve the residue in petroleum ether, acidify twice with 3M sulfuric acid, wash with water until neutral, remove the solvent, and dry to obtain 18.9g of product...
Embodiment 2
[0126] Example 2: Synthesis of 1,2-dimethyl-1-propylphosphonic acid monoisooctyl ester
[0127] Add 10.6 g of sodium phosphinate monohydrate, 80 mL of DMF, 7.0 g of isopentene, and 4.8 g of benzoyl peroxide into a three-necked flask in sequence, heat to 110 degrees under normal pressure, and reflux for 2 hours. After adding the initiator benzoyl peroxide 4.8g, the reflux reaction was continued for 2 hours. After adding the initiator benzoyl peroxide 4.8g again, the reflux reaction was continued for 2 hours. Wash twice with water, once with saturated brine, and extract twice with ether. Combine the organic phases, wash with water, dry over anhydrous sodium sulfate, filter with suction, and remove the solvent under reduced pressure to obtain 29.4 g of the product with a yield of 78.2%. Add 55ml of ethanolamine, heat and reflux for 30 hours, recover the excess ethanolamine under reduced pressure, dissolve the residue in petroleum ether, acidify twice with 3M sulfuric acid, wash ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com