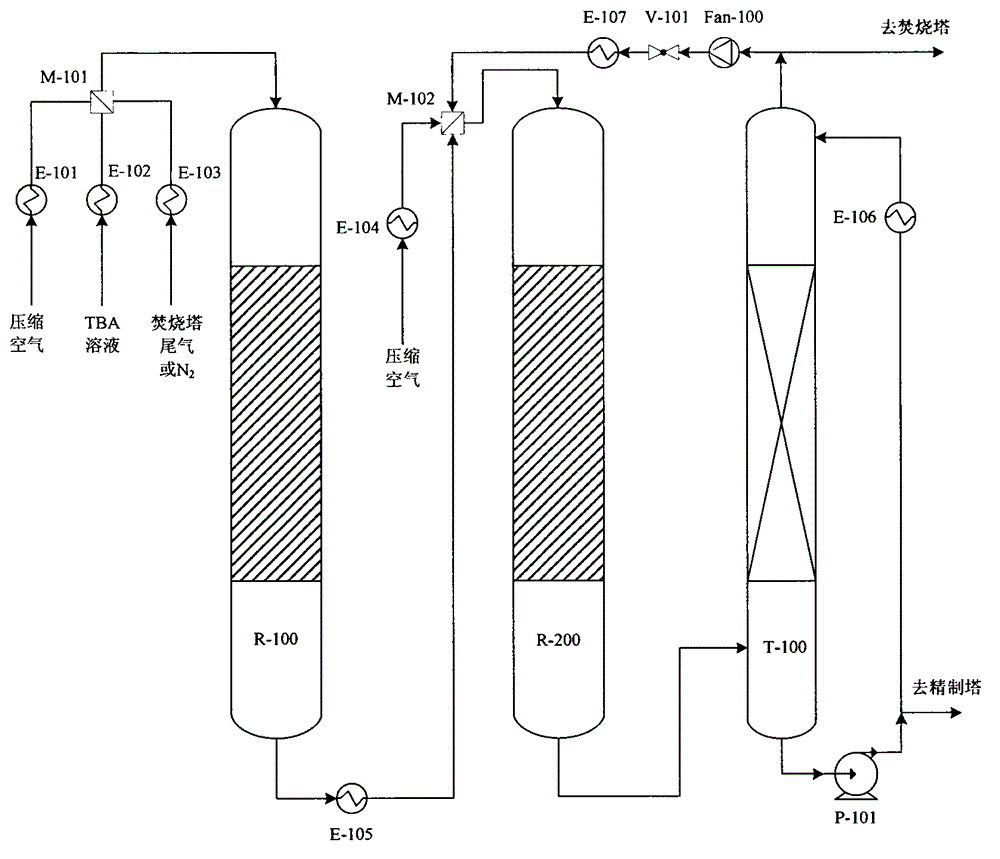
Utilization method of tertiary butyl alcohol coproduced by using device for producing epoxypropane by using propene and iso-butane cooxidation method
A technology of co-oxidation and propylene oxide, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems that tert-butanol cannot be used directly, affects product economy, and increases cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
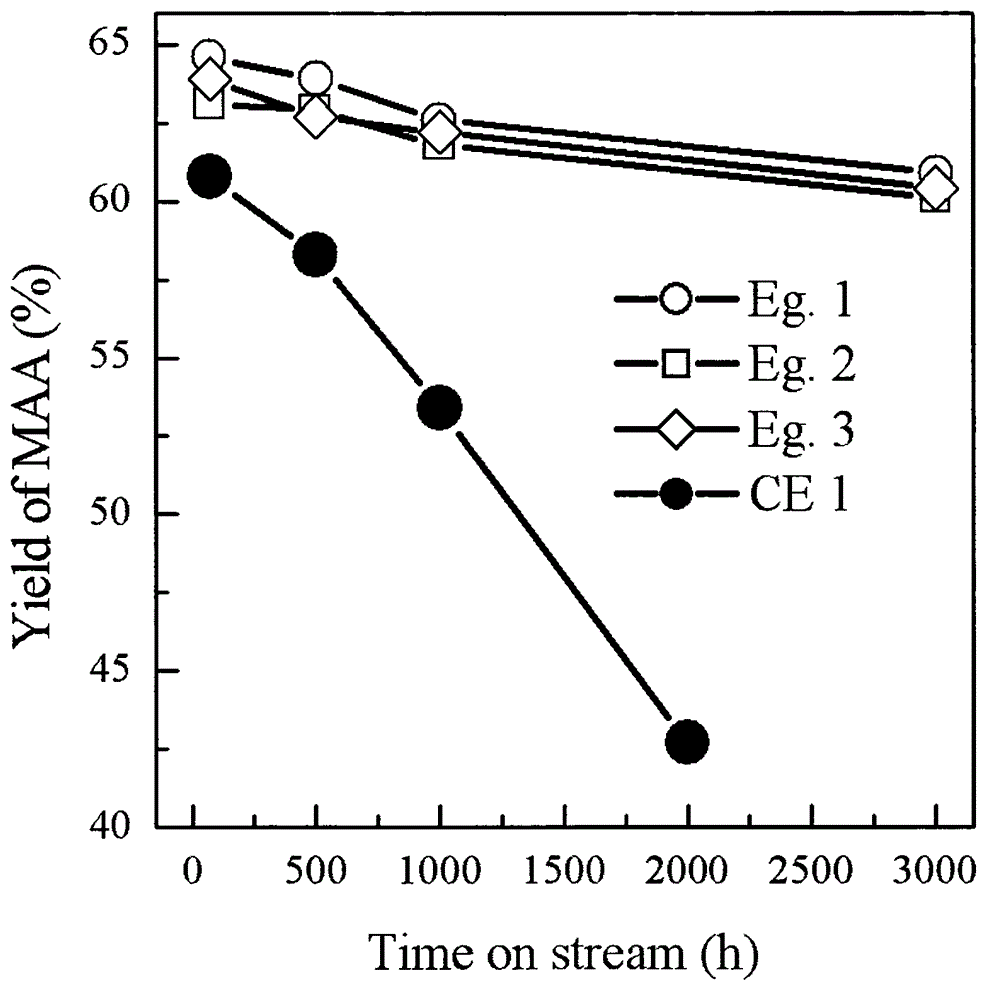
Image
Examples
Embodiment 1
[0058] Catalyst preparation:
[0059] Preparation of the first stage catalyst:
[0060] Add 449 parts of ammonium heptamolybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O, molecular weight 1236) and 22.3 parts of ammonium metavanadate (NH 4 VO 3 , molecular weight: 117), heated up to 80°C while stirring, and added 339 parts of bismuth nitrate (Bi(NO 3 ) 3 .5H 2 O, molecular weight 485), 317 parts of iron nitrate (Fe(NO 3 ) 3 9H 2 O, molecular weight 404), 111 parts of cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O, molecular weight 291), 20.5 parts of copper nitrate (Cu(NO 3 ) 2 ·3H 2 O, molecular weight 242) and 20.7 parts cesium carbonate (Cs 2 CO 3 , molecular weight 326) was dissolved in 370 parts of nitric acid solution with a mass concentration of 10%, continued to stir for 30 minutes, then added ammonia water to adjust the pH to 3.0, aged the slurry for 10 hours and dried it in a blast drying oven at 90°C for 12 hours, and finally the catalyst After being ground into fine po...
Embodiment 2
[0076] Catalyst preparation:
[0077] Preparation of the first stage catalyst:
[0078] Add 449 parts of ammonium heptamolybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O, molecular weight 1236), 11.2 parts niobium pentoxide (Nb 2 o 5 , molecular weight: 265.8) and 7.4 parts of ammonium metavanadate (NH 4 VO 3 , molecular weight: 117), heated up to 80°C while stirring, and added 102.7 parts of bismuth nitrate (Bi(NO 3 ) 3 .5H 2 O, molecular weight 485), 599 parts of iron nitrate (Fe(NO 3 ) 3 9H 2 O, molecular weight 404), 123.3 parts of cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O, molecular weight 291), 102.5 parts of copper nitrate (Cu(NO 3 ) 2 ·3H 2 O, molecular weight 242) and 13.8 parts cesium carbonate (Cs 2 CO 3 , molecular weight 326) was dissolved in 370 parts of nitric acid solution with a mass concentration of 10%, continued to stir for 30 minutes, then added ammonia water to adjust the pH to 3.0, aged the slurry for 10 hours and dried it in a blast drying oven at 90°...
Embodiment 3
[0095] Catalyst preparation:
[0096] Preparation of the first stage catalyst:
[0097] Add 449 parts by weight ammonium heptamolybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O, molecular weight 1236), 5.6 parts niobium pentoxide (Nb 2 o 5 , molecular weight: 265.8) and 99.0 parts of ammonium metavanadate (NH 4 VO 3 , Molecular weight: 117), heated up to 80°C while stirring, and added 152.5 parts of bismuth nitrate (Bi(NO 3 ) 3 .5H 2 O, molecular weight 485), 171.2 parts of iron nitrate (Fe(NO 3 ) 3 9H 2 O, molecular weight 404), 246.4 parts of cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O, molecular weight 291), 76.9 parts of copper nitrate (Cu(NO 3 ) 2 ·3H 2 O, molecular weight 242) and 51.8 parts cesium carbonate (Cs 2 CO 3 , molecular weight 326) was dissolved in 370 parts of nitric acid solution with a mass concentration of 10%, continued to stir for 30 minutes, then added ammonia water to adjust the pH to 3.0, aged the slurry for 10 hours and dried it in a blast drying ove...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com