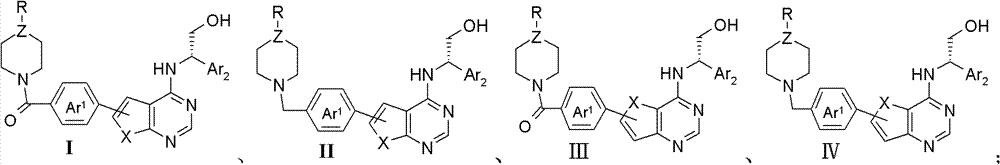
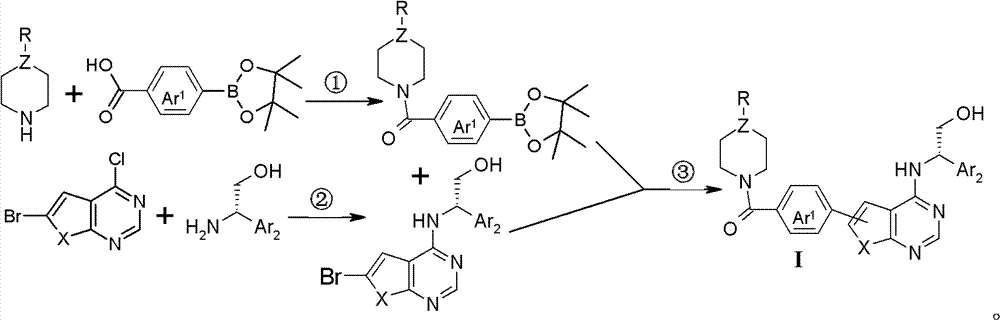
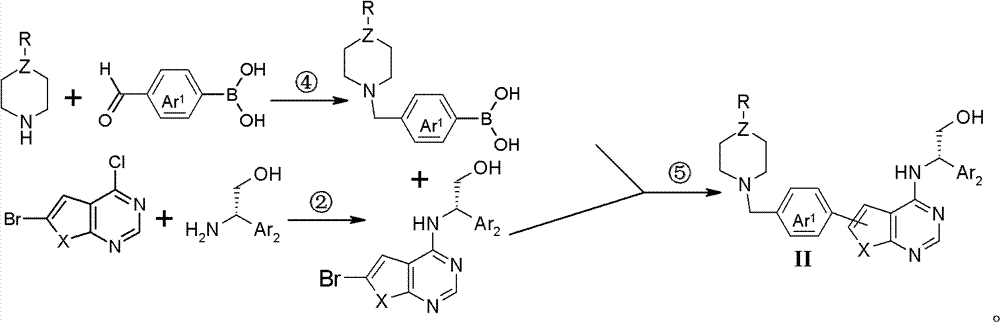
Thienopyrimidine and furopyrimidine derivative, its preparation method and application in medicines
A technology for pyrimidine derivatives, which is applied in the field of pyrimidine derivatives and their preparation, can solve the problems of low drug resistance and achieve the effects of low drug resistance, novel structure, and obvious EGFR inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: Preparation of compound I-1 and compound II-1
[0054]
[0055] first step:
[0056] Dissolve p-carboxyphenylboronic acid pinacol ester (1g, 4.03mmol) in dichloromethane (10ml, 0.16mol) and N,N-dimethylformamide (1ml, 13.0mmol) at room temperature, add N-ethyl Piperazine (0.6ml, 4.8mmol), and sequentially added 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (0.93g, 4.8mmol), N-hydroxybenzo Triazole (0.66g, 4.8mmol), triethylamine (0.84ml, 6.04mmol), stirring reaction at room temperature until TLC monitoring raw material reaction is complete, in reaction solution, add 10ml water, stir 30 minutes, dichloromethane (50ml *3) extraction, and then washed with saturated sodium chloride solution (50ml*2), the organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain: (4-ethyl-piperazin-1-yl)- [4-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-methanone (1.4 g, pale yellow solid), d...
Embodiment 2
[0070] Embodiment 2: preparation compound I-2 and compound II-2
[0071]
[0072] first step:
[0073] P-carboxyphenylboronic acid pinacol ester (3g, 12.10mmol) was dissolved in dichloromethane (27ml, 0.422mol) and N,N-dimethylformamide (9ml, 0.116mol) at room temperature, and N-formaldehyde was added Piperazine (1.45g, 14.5mmol), and successively add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (2.79g, 14.4mmol), N-hydroxybenzo Triazole (1.98g, 12.9mmol), triethylamine (2.5ml, 17.99mmol), stirring reaction at room temperature until TLC monitoring raw material reaction is complete, in reaction solution, add 30ml water, stir 30 minutes, dichloromethane (100ml *3) extraction, and then washed with saturated sodium chloride solution (100ml*2), the organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain: (4-methyl-piperazin-1-yl)- [4-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-methanone (1.5...
Embodiment 3
[0082] Embodiment 3: preparation compound I-3 and compound II-3
[0083]
[0084] first step:
[0085] P-carboxyphenylboronic acid pinacol ester (3g, 12.10mmol) was dissolved in dichloromethane (27ml, 0.422mol) and N,N-dimethylformamide (9ml, 0.116mol) at room temperature, and 1-cyclo Propylmethylpiperazine (2.3g, 14.9mmol), and successively added 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (2.79g, 14.4mmol), N- Hydroxybenzotriazole (1.98g, 12.9mmol), triethylamine (2.5ml, 17.99mmol), the reaction was stirred at room temperature until the reaction of the raw materials monitored by TLC was complete, 30ml of water was added to the reaction solution, stirred for 30 minutes, and dichloro Extracted with methane (100ml*3), washed with saturated sodium chloride solution (100ml*2), dried the organic phase with anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain: (4-cyclopropylcarbonyl-piperazine- 1-yl)-[4-(4,4,5,5-tetramethyl-[1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com