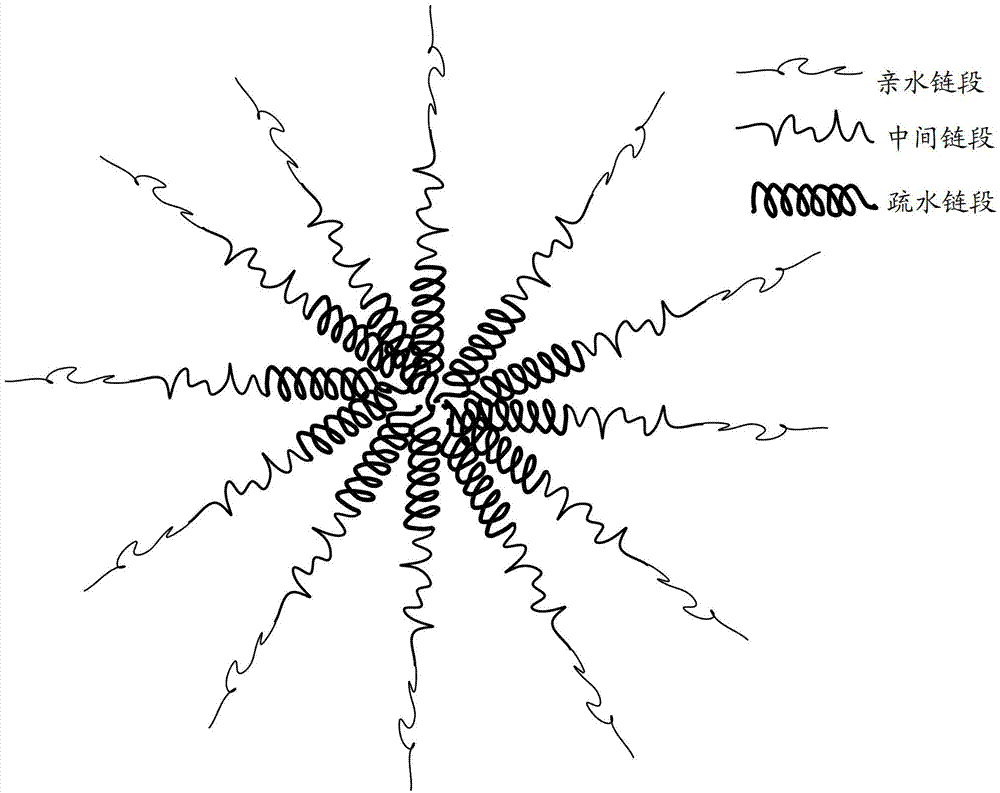
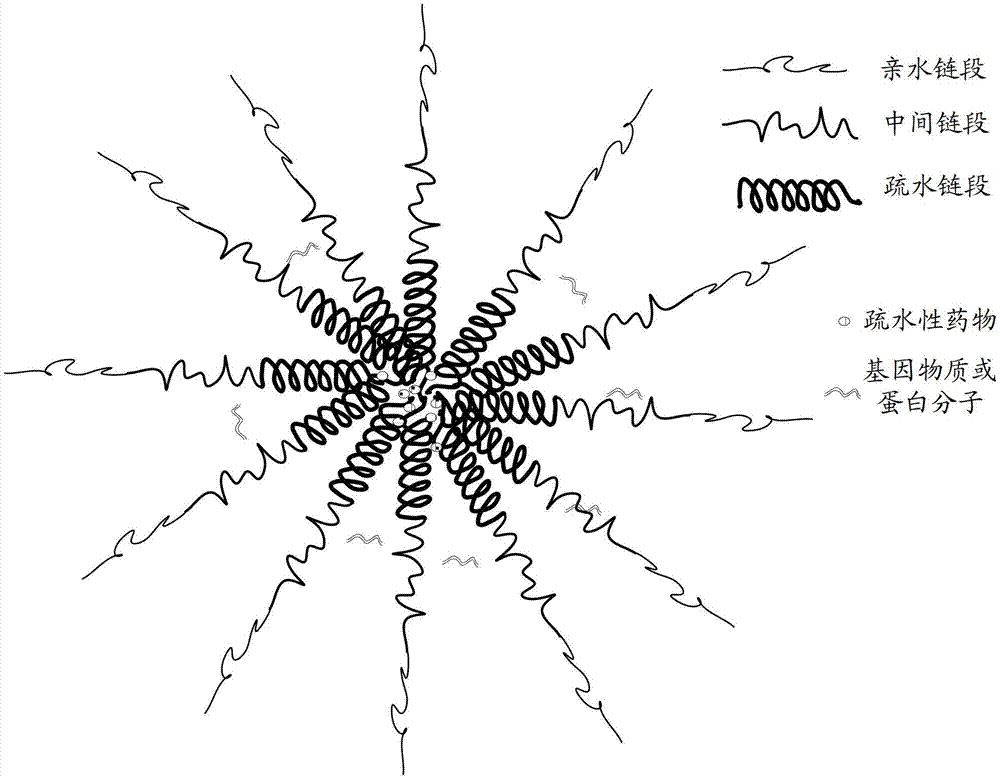
Amphipathy triblock polymer and preparation method and application thereof
A polymer, triblock technology, applied in the field of nanomedicine, can solve the problems of single function and limited application, and achieve the effect of wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] In addition, this embodiment also provides a method for preparing an amphiphilic tri-block polymer, comprising the following steps:
[0056] Step 110, under a protective gas atmosphere, dissolving amino acid monomers and polyethylene glycol derivatives in N,N-dimethylformamide to obtain a mixed solution, and then reacting at a constant temperature at 30-50°C for 24-120 hours , wherein the amino acid monomer is formed by the internal anhydride of leucine (Leu-NCA), the internal anhydride of lysine containing a side chain protecting group, and the internal anhydride of glutamic acid containing a side chain protecting group The mixture, the ratio of the total moles of the mixture to the moles of the polyethylene glycol derivative is 10:1 to 200:1, and the concentration of the polyethylene glycol derivative in the mixture is 1 to 100 mg / mL.
[0057] Step 120, adding phenylalanine cyclic anhydride (Phe-NCA) to the reacted mixed solution, and reacting at a constant temperatur...
Embodiment 1
[0083] Preparation of P1 Amphiphilic Triblock Polymer
[0084]The closed round-bottomed flask was evacuated and filled with nitrogen; then 1 g of monomethyl ether amino polyethylene glycol (MPEG) with a molecular weight of 2000 was dissolved in 20 mL of N,N-dimethylformamide (DMF), and added into the above-mentioned round-bottomed flask; then add Lys(Z)-NCA, Glu(OBzl)-NCA and Leu-NCA three monomers to the above-mentioned round-bottomed flask, and react at a constant temperature of 40°C for 72 hours under nitrogen protection; After the end, add Phe-NCA monomer to the above-mentioned round-bottomed flask, and react at a constant temperature of 40°C for 48 hours under the protection of nitrogen; The amphiphilic triblock polymer of side chain protecting group, MPEG-b-P[Lys(Z)-co-Leu-co-Glu(OBzl)]-b-PPhe; Wherein, the total molar number of three kinds of monomers and The molar ratio of monomethyl ether amino polyethylene glycol is 30:1, and the molar ratio of Phe-NCA monomer to mo...
Embodiment 2
[0088] Preparation of P2 Amphiphilic Triblock Polymer
[0089] The closed round bottom flask was evacuated and filled with nitrogen; then 0.5 g of aminopolyethylene glycol carboxylic acid (NH 2 -PEG-COOH) was dissolved in 15mL DMF and added to the above round bottom flask; then three monomers, Lys(Z)-NCA, Glu(OBzl)-NCA and Leu-NCA were added to the above round bottom flask, Under the protection of nitrogen, react at a constant temperature of 30°C for 72 hours; after the reaction, add Phe-NCA monomer to the above-mentioned round bottom flask, and react at a constant temperature of 30°C for 72 hours under the protection of nitrogen; after the reaction, add 15 times The diethyl ether of the volume of reaction solution is precipitated, centrifuged, and dried to obtain an amphiphilic triblock polymer containing side chain protecting groups, HOOC-PEG-b-P[Lys(Z)-co-Leu-co-Glu(OBzl)]- b-PPhe; wherein, the ratio of the total moles of the three monomers to the moles of the aminopolyeth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com