Main-chain benzoxazine oligomer compositions, and method for the preparation thereof
A benzoxazine and oligomer technology, applied in the field of main chain benzoxazine compositions, can solve the problems of product insolubility, low molecular weight, wide polydispersity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of a mixture of bisphenol-F benzoxazine monomers
[0055] In a 250 ml round bottom flask, bisphenol F isomer (80 mmol, 16.02 g) and aniline (160 mmol, 14.90 g) were dissolved in 75 ml of toluene. Paraformaldehyde (320 mmol, 9.60 g) was added to the solution, and then the mixture was heated at 90°C in a preheated oil bath. After the reaction, pass 1 H NMR found that the best conversion to the cyclic benzoxazine structure was achieved after about 5 hours. The solution was cooled and precipitated in hexane to obtain a yellow powder. Finally, the powder was placed in a vacuum oven at 40°C for 72 hours to dry (yield: 32 g, 81%).
Embodiment 2
[0056] Example 2: Preparation of a mixture of main chain benzoxazine oligomers
[0057] A mixture of bisphenol F isomer (100 mmol, 20.02 g), aniline (100 mmol, 9.13 g), DDM (50 mmol.9.90 g) and paraformaldehyde (400 mmol, 12 g) was added with 100 mL as a solvent Of toluene in a 250 ml round bottom flask. The milky mixture was gradually heated and kept stirring at 90°C for 5 hours. After about 15 minutes, the formation of a triaza gel was observed. After 3 hours, the insoluble white gel disappeared, forming a transparent yellow solution. Under the same conditions, the reaction mixture was stirred for another 3 hours. Then it was cooled to room temperature, and then poured into hexane to obtain a yellow powder. The powder was re-dissolved in tetrahydrofuran and reprecipitated in methanol to produce a light yellow powder (yield: 36 g, 71%).
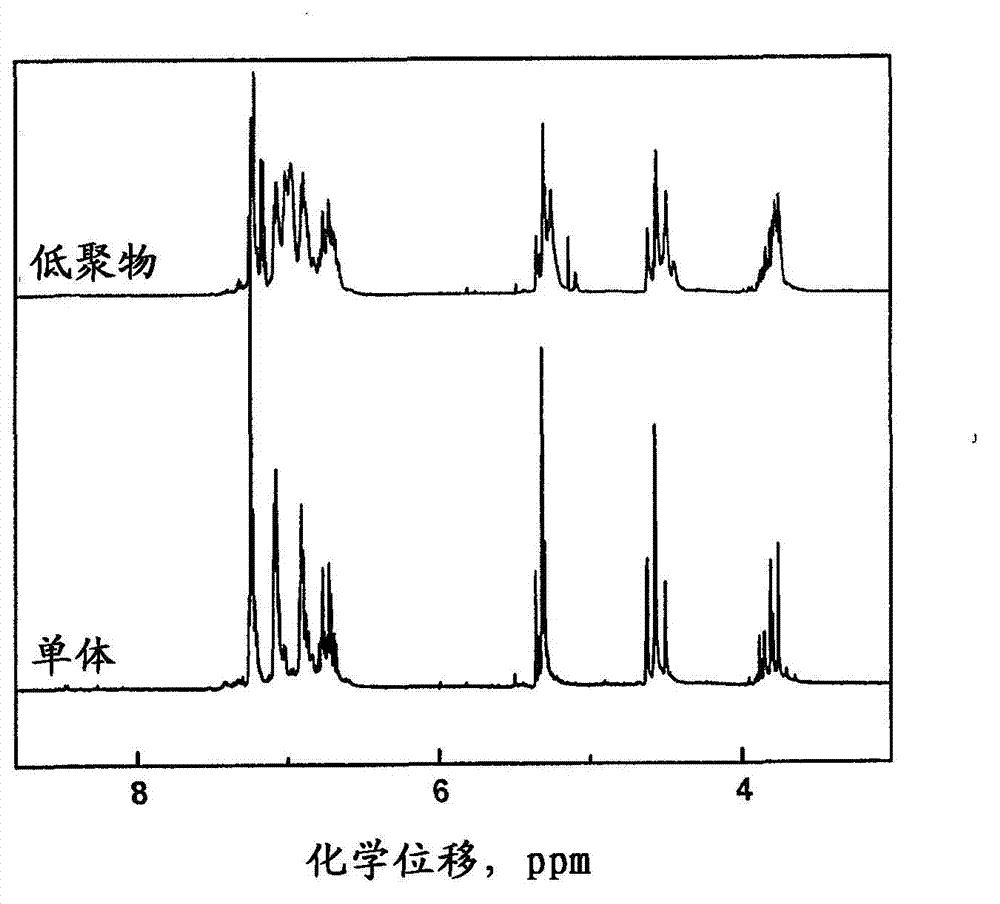
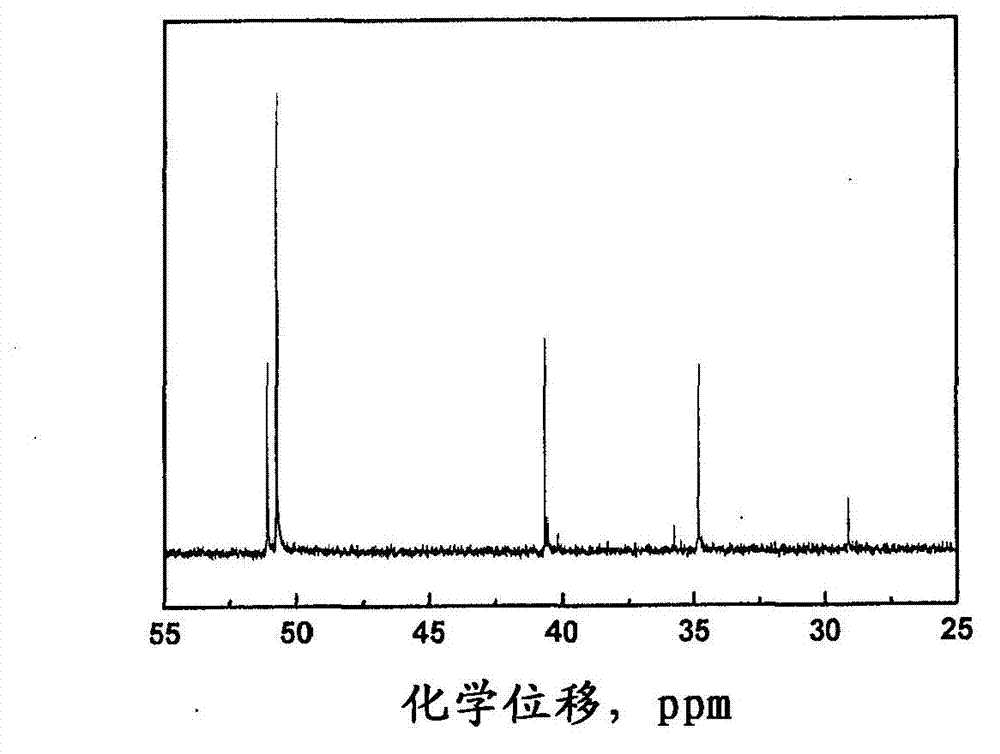
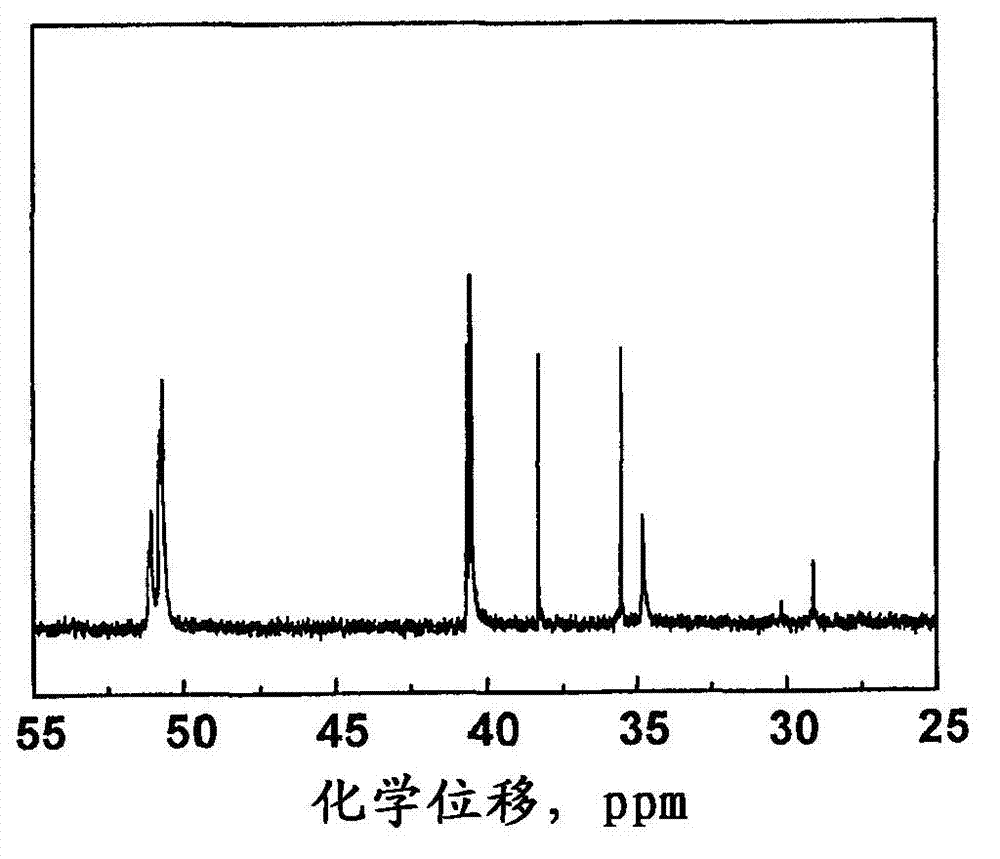
[0058] figure 1 Show the benzoxazine monomers and main chain oligomers prepared in Examples 1 and 2 1 H-NMR spectrum. Usually, due to the ...
Embodiment 3
[0064] Example 3: Preparation of benzoxazine blend for viscosity measurement
[0065] Blends of monomers and oligomers were prepared in chloroform with monomer to oligomer ratios of 10%, 30%, and 50%, and then placed in a vacuum oven at 40°C for 72 hours to remove the solvent. by 1 H-NMR inspects dried samples to evaluate the drying of the solvent used, and only those samples showing negligible solvent content are used for viscosity testing.
[0066] Figure 4 Shows the dynamic viscosity of benzoxazine (including pure oligomers, monomers and oligomer blends). Low-viscosity benzoxazine monomers can be used as reactive diluents. In the mixture of benzoxazine monomers and oligomers, both benzoxazines can be polymerized through a ring-opening reaction to produce cross-linked products. In addition, by mixing the benzoxazine monomer and the benzoxazine oligomer, the viscosity of the mixture can be adjusted and controlled by changing the ratio of the two benzoxazines in the mixture. Fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com