Preparation method of capsule containing tegafur, gimeracil and potassium oxonate
A technology for oteracil potassium and capsules is applied in the field of preparation of capsules, which can solve the problems of gastrointestinal irritation, affect the physiological activity of preparations and the like, and achieve the advantages of reducing irritation, improving bioavailability and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
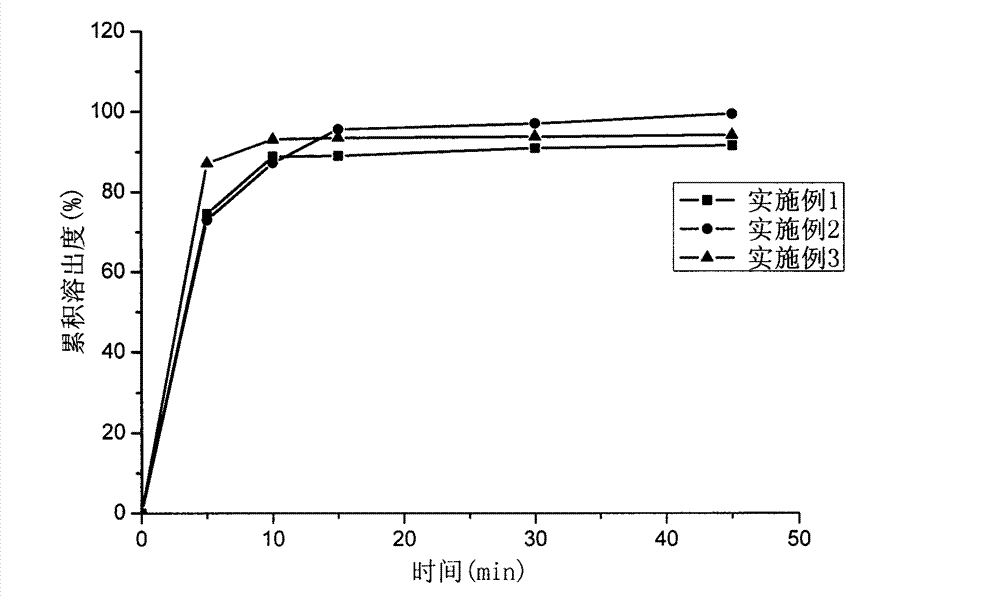
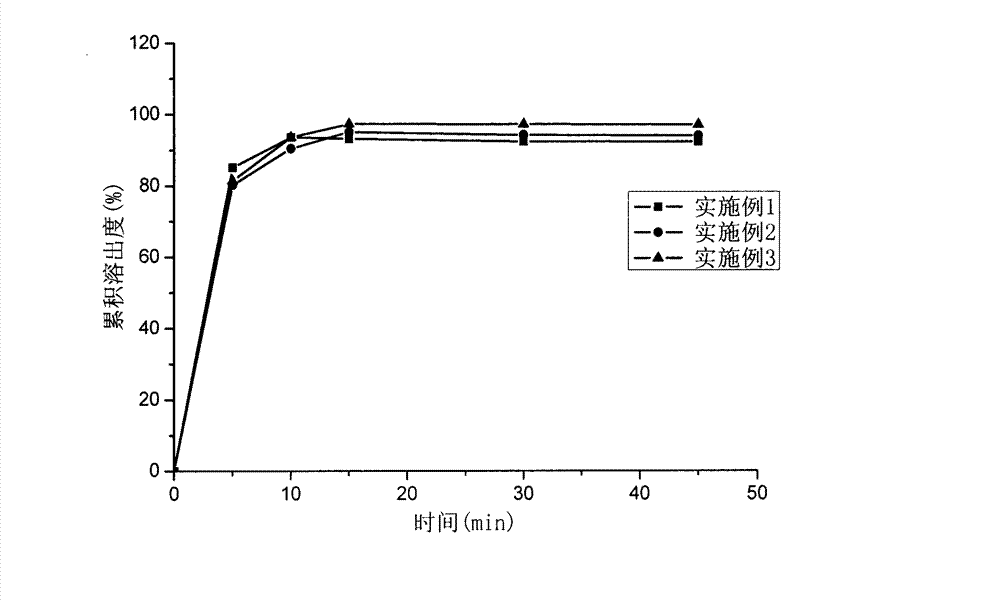
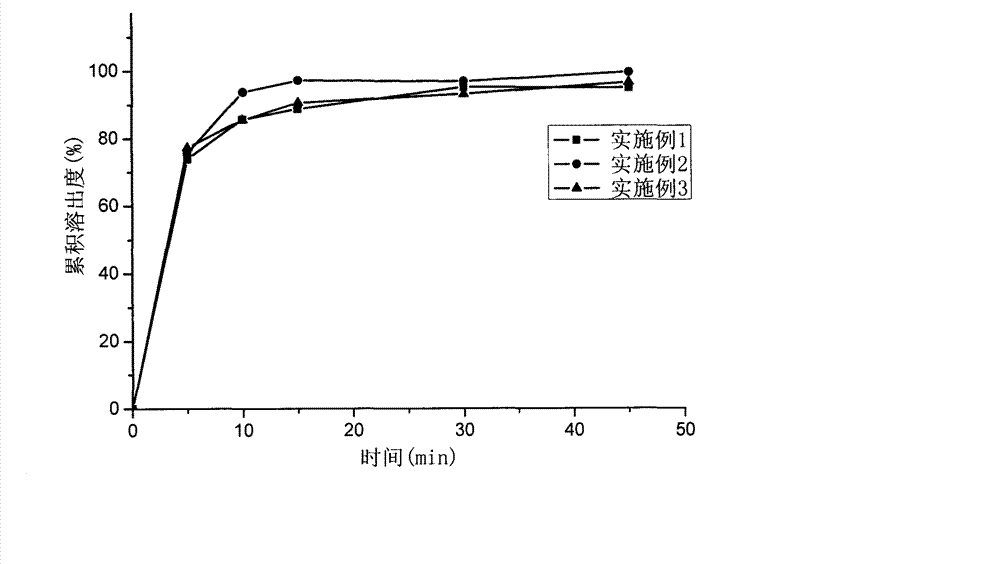
Examples
Embodiment 1
[0026] The composition of the prescription is as follows (based on 1000 capsules):
[0027]
[0028] Preparation Process:
[0029] (1) Tegafur, oteracil potassium, and gimeracil are respectively passed through a 100-mesh sieve, microcrystalline cellulose and pregelatinized starch are respectively passed through a 80-mesh sieve, and magnesium stearate is passed through a 120-mesh sieve for subsequent use;
[0030] (2) The gemeracil and the pregelatinized starch that take prescription quantity are mixed uniformly, carry out air-flow micronization, obtain gemeracil-pregelatinized starch mixture micropowder;
[0031] (3) Weigh Tegafur, Oteracil Potassium, and Microcrystalline Cellulose in the prescribed amount, mix them with the above-mentioned mixture micropowder, and add 2% PVP K30 Soft material made from ethanol solution, granulated with a 26-mesh sieve, dried at 60°C for 3 hours, granulated with a 24-mesh sieve;
[0032] (4) Take the above granules, add the prescribed am...
Embodiment 2
[0034] The composition of the prescription is as follows (based on 1000 capsules):
[0035]
[0036] Preparation Process:
[0037] (1) Tegafur, oteracil potassium, and gimeracil were respectively passed through a 100-mesh sieve, lactose, microcrystalline cellulose, and pregelatinized starch were respectively passed through a 80-mesh sieve, and magnesium stearate was passed through a 120-mesh sieve for subsequent use;
[0038] (2) The gemeracil and lactose of prescription quantity are weighed and mixed homogeneously, put in the ball mill and take out after ball milling for 1h, obtain the gemeracil-lactose mixture micropowder;
[0039] (3) Weigh Tegafur, Oteracil Potassium, Microcrystalline Cellulose, Pregelatinized Starch, mix with the above-mentioned mixture micropowder, add 5% PVP K30 Soft material made from ethanol solution, granulated with a 26-mesh sieve, dried at 60°C for 2 hours, granulated with a 24-mesh sieve;
[0040] (4) Take the above granules, add the prescr...
Embodiment 3
[0042] The composition of the prescription is as follows (based on 1000 capsules):
[0043]
[0044]
[0045] Preparation Process:
[0046] (1) Tegafur, oteracil potassium, and gimeracil were respectively passed through a 100-mesh sieve, microcrystalline cellulose and pregelatinized starch were respectively passed through a 80-mesh sieve, and magnesium stearate was passed through a 120-mesh sieve for subsequent use;
[0047] (2) the gemeracil and 40g pregelatinized starch that take prescription quantity are mixed homogeneously, carry out air-flow micronization, obtain gemeracil-pregelatinized starch mixture micropowder;
[0048] (3) Take tegafur, oteracil potassium, and microcrystalline cellulose of prescription quantity, mix with the above-mentioned mixture micropowder and remaining pregelatinized starch, add 4% hypromellose aqueous solution to make soft material, Granulate with a 26-mesh sieve, dry at 60°C for 3 hours, and granulate with a 24-mesh sieve;
[0049] (4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com