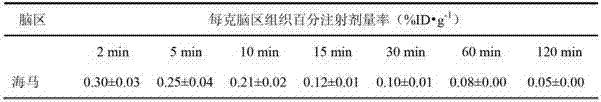
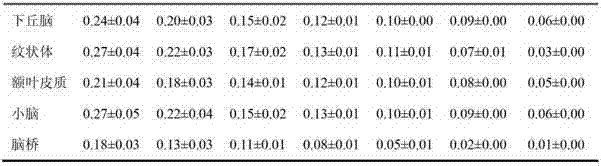
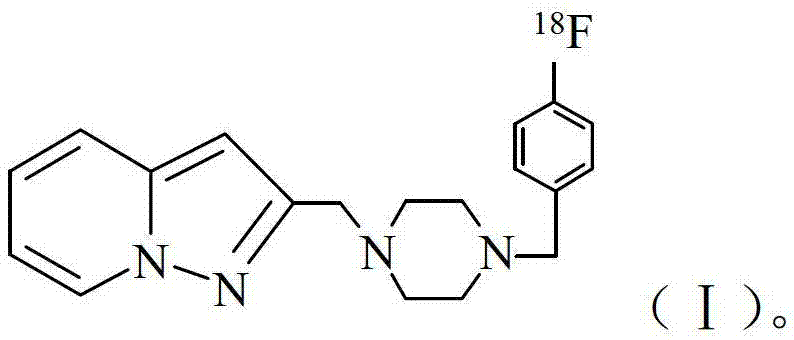
Fluoride-18 marked dopamine D4 receptor developing agent and preparation method thereof
A technology of dopamine and imaging agent, which is applied in the fields of chemistry and medicine, can solve the problem of small specificity, and achieve the effect of high product yield, high purity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: 2-[4-(4-[ 18 Synthesis of F]fluorobenzyl)piperazin-1-ylmethyl]pyrazolo[1,5-α]pyridine
[0020] (1) Synthesis of 2-[4-piperazinecarbaldehyde-1-ylmethyl]pyrazolo[1,5-α]pyridine
[0021] Dissolve 0.1465g (1.000mmol) of pyrazolo[1,5-α]pyridine-2-carbaldehyde and 0.1712g (1.499mmol) of N-formylpiperazine in 10mL of dichloromethane, and add sodium triacetoxyborohydride 0.6782g (3.242mmol), stirred at room temperature for 24 hours, added ice water, mixed well, extracted with dichloromethane, dried the organic layer with anhydrous sodium sulfate, separated and purified by column chromatography to obtain yellow solid 2-[4-piper Azinecarbaldehyde-1-ylmethyl]pyrazolo[1,5-α]pyridine 0.1048g, yield 42.9%. Mp116.9°C. 1 H NMR (CDCl 3 ,500MHz)δ:2.69(br s,4H,-(CH 2 ) 2 N piperazinyl);3.18(br s,4H,-(CH 2 ) 2 Npiperazinyl); 3.85(br s,2H,-CH 2 );6.49(s,1H,3-H);6.72(dd,J=7.3Hz,1H,6-H);7.05(dd,J=7.2Hz,1H,5-H);7.50(br d, J=8.5Hz,1H,4-H);7.82(s,1H,-CHO);8.41(dd,J=7.0Hz,J=1...
Embodiment 2
[0032] Example 2: 2-[4-(4-[ 18 Synthesis of F]fluorobenzyl)piperazin-1-ylmethyl]pyrazolo[1,5-α]pyridine
[0033] (1) Synthesis of 2-[4-piperazinecarbaldehyde-1-ylmethyl]pyrazolo[1,5-α]pyridine
[0034] Dissolve 0.1465g (1.000mmol) of pyrazolo[1,5-α]pyridine-2-carbaldehyde and 0.3420g (2.996mmol) of N-formylpiperazine in 10mL of acetonitrile, and add 0.6750g of sodium triacetoxyborohydride (3.185mmol), after stirring at room temperature for 20 hours, add ice water, mix well and extract with acetonitrile, the organic layer is dried with anhydrous sodium sulfate, separated and purified by column chromatography to obtain yellow solid 2-[4-piperazine formaldehyde-1 -ylmethyl]pyrazolo[1,5-α]pyridine 0.1312g.
[0035] (2) Synthesis of 2-[4-piperazin-1-ylmethyl]pyrazolo[1,5-α]pyridine
[0036] 2-[4-piperazine formaldehyde-1-ylmethyl]pyrazolo[1,5-α]pyridine (0.1000g, 0.4093mmol) and 6mol / L H 2 SO 4 (10mL) reacted at 60°C for 10 hours, cooled to room temperature, added concentrated...
Embodiment 3
[0041] Example 3: In vitro receptor binding analysis of 2-[4-(4-fluorobenzyl)piperazin-1-ylmethyl]pyrazolo[1,5-α]pyridine
[0042] Take 33 test tubes (12×60mm) and divide them into 3 groups: total binding tube (TB tube, 1×3), non-specific binding tube (NSB tube, 1×3), sample tube (SB tube, 9×3 branch). Add [ 3 H]Spiperone (spiperone) (the final concentration is 0.7nmol / L), add 100 μL of buffer solution to the total binding tube, add (+)-Butaclamol hydrochloride (butaclamol hydrochloride) 20 μL to the non-specific binding tube ( The final concentration is 1 μM), and a series of concentrations of 2-[4-(4-fluorobenzyl)piperazin-1-ylmethyl]pyrazolo[1,5-α]pyridine dissolved in DMF and water were added to the sample tube (Ligand) (The final concentration of each tube is 1×10 -11 mol / L, 1×10 -10 mol / L, 1×10 -9 mol / L, 1×10 -8 mol / L, 1×10 -7 mol / L, 1×10 -6 mol / L, 1×10 -5 mol / L, 1×10 -4 mol / L, 1×10 -3 mol / L), various reagents and dopamine receptors were added according to Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com